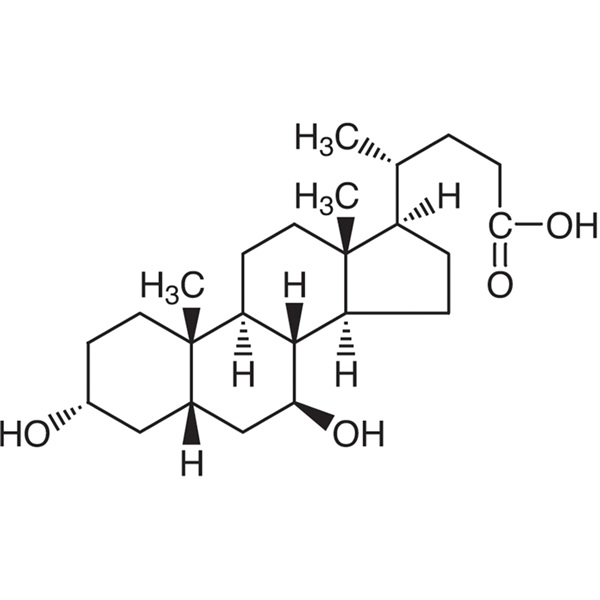
Ursodeoxycholic Acid (UDCA) CAS 128-13-2 Assay 99.0~101.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Ursodeoxycholic Acid (UDCA; Ursodiol) (CAS: 128-13-2) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Ursodeoxycholic Acid, Please contact: alvin@ruifuchem.com
| Chemical Name | Ursodeoxycholic Acid |
| Synonyms | UDCA; Ursodiol; 3α,7β-Dihydroxy-5β-Cholan-24-oic Acid; 5β-Cholanic Acid-3α,7β-diol; 3α,7β-Dihydroxy-5β-Cholanic Acid; (3α,5β,7β)-3,7-Dihydroxycholan-24-oic acid; 7β-Hydroxylithocholic Acid; UDCS; (3alpha,5beta,7beta)-3,7-Dihydroxy-cholan-24-oic Acid |
| Stock Status | In Stock, Production Capacity 120 Tons per Year |
| CAS Number | 128-13-2 |
| Molecular Formula | C24H40O4 |
| Molecular Weight | 392.58 g/mol |
| Melting Point | About 202.0℃ |
| Density | 1.128g/cm3 |
| Solubility | Practically Insoluble in Water, Freely Soluble in Ethanol (96 percent), Slightly Soluble in Acetone, Practically Insoluble in Methylene Chloride |
| Solubility in EtOH | Almost Transparency |
| COA & MSDS | Available |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Almost White Powder | White Powder |
| Identification: A | Infrared Absorption Spectrophotometry | Complies |
| Identification: B | Thin-Layer Chromatography Test for Impurity C | Complies |
| Identification: C | The Suspension Obtained is Greenish-Blue | Complies |
| Melting Point | About 202.0℃ | 202.3℃ |
| Specific Rotation [a]20/D | +58.0°~+62.0° (C=4 in EtOH) | +59.3° |
| Mesh Size | 80 Mesh | 80 Mesh |
| Loss on Drying | ≤1.00% | 0.48% |
| Sulfated Ash | ≤0.10% | <0.10% |
| Impurity C | ≤0.10% (Lithocholic Acid) | <0.10% |
| Related Substances | ||
| Impurity A | ≤1.00% (Chenodeoxycholic Acid) | <1.00% |
| Unspecified Impurities | ≤0.10% | <0.10% |
| Total Impurities | ≤1.50% | <1.50% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Microbiological Limit Test | ||
| Total Plate Count | ≤1000cfu/g | Complies |
| Total Yeasts & Molds | ≤100cfu/g | Complies |
| Salmonella | Negative | Negative |
| Escherichia Coli | Negative | Negative |
| Assay | 99.0~101.0% (Dried Substance) | 99.98% |
| Conclusion | The product has been tested and complies with the given specifications | |
| Shelf Life | 36 Months Under Well Storage Conditions | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from strong sunlight, heat, moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
URSODEOXYCHOLIC ACID
C24H40O4 Mr 392.6 [128-13-2]
DEFINITION
3α,7β-Dihydroxy-5β-cholan-24-oic acid.
Content: 99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance: white or almost white powder.
Solubility: practically insoluble in water, freely soluble in ethanol (96 per cent), slightly soluble in acetone, practically insoluble in methylene chloride.
mp: about 202℃.
IDENTIFICATION
First identification: A.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ursodeoxycholic Acid CRS.
B. Examine the chromatograms obtained in the test for impurity C.
Results: the principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve about 10 mg in 1 mL of sulfuric acid R. Add 0.1 mL of formaldehyde solution R and allow to stand for 5 min. Add 5 mL of water R. The suspension obtained is greenish-blue.
TESTS
Specific optical rotation (2.2.7): + 58.0 to + 62.0 (dried substance).
Dissolve 0.500 g in anhydrous ethanol R and dilute to 25.0 mL with the same solvent.
Impurity C. Thin-layer chromatography (2.2.27).
Solvent mixture: water R, acetone R (10:90 V/V).
Test solution (a). Dissolve 0.40 g of the substance to be examined in the solvent mixture and dilute to 10 mL with the solvent mixture.
Test solution (b). Dilute 1 mL of test solution (a) to 10 mL with the solvent mixture.
Reference solution (a). Dissolve 40 mg of ursodeoxycholic acid CRS in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution (b). Dissolve 20 mg of lithocholic acid CRS (impurity C) in the solvent mixture and dilute to 10.0 mL with the solvent mixture (solution A). Dilute 2.0 mL of this solution to 100.0 mL with the solvent mixture.
Reference solution (c). To 5 mL of solution A add 10 mg of chenodeoxycholic acid CRS (impurity A) and dilute to 50 mL with the solvent mixture.
Plate: TLC silica gel plate R.
Mobile phase: glacial acetic acid R, acetone R, methylene chloride R (1:30:60 V/V/V).
Application: 5μL.
Development: over 2/3 of the plate.
Drying: at 120℃ for 10 min.
Detection: spray immediately with a 47.6 g/L solution of phosphomolybdic acid R in a mixture of 1 volume of sulfuric acid R and 20 volumes of glacial acetic acid R and heat at 120℃ until blue spots appear on a lighter background.
System suitability: reference solution (c):
- the chromatogram shows 2 clearly separated principal spots.
Limit: test solution (a):
-impurity C: any spot due to impurity C is not more intense than the principal spot in the chromatogram obtained with reference solution (b) (0.1 per cent).
Related substances. Liquid chromatography (2.2.29).
Solvent mixture: methanol R, mobile phase (10:90 V/V).
Test solution. Dissolve 60 mg of the substance to be examined in the solvent mixture and dilute to 20.0 mL with the solvent mixture.
Reference solution (a). Dissolve the contents of a vial of Ursodeoxycholic Acid for system suitability CRS (containing impurities A and H) in 1.0 mL of the solvent mixture.
Reference solution (b). Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Column:
-size: l = 0.25 m, Ø = 4.6 mm;
-stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
- temperature: 40℃ ± 1℃.
Mobile phase: mix 30 volumes of acetonitrile R, 37 volumes of a 0.78 g/L solution of sodium dihydrogen phosphate R adjusted to pH 3 with phosphoric acid R, and 40 volumes of methanol R.
Flow rate: 0.8 mL/min.
Detection: refractometer at 35 ± 1℃.
Injection: 150 μL.
Run time: 4 times the retention time of Ursodeoxycholic Acid.
Identification of impurities: use the chromatogram supplied with ursodeoxycholic acid for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A and H.
Relative retention with reference to ursodeoxycholic acid (retention time = about 14 min): impurity H = about 0.9; impurity A = about 2.8.
System suitability: reference solution (a):
-resolution: minimum 1.5 between the peaks due to impurity H and ursodeoxycholic acid.
Limits:
- impurity A: not more than 10 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 percent);
-unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
- total: not more than 15 times the area of the principal peak in the chromatogram obtained with reference solution (b) (1.5 percent);
-disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 percent).
Heavy metals (2.4.8): maximum 20 ppm.
1.0 g complies with test C. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Loss on drying (2.2.32): maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105℃.
Sulfated ash (2.4.14): maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.350 g in 50 mL of ethanol (96 per cent) R, previously neutralised to 0.2 mL of phenolphthalein solution R. Add 50 mL of water R and titrate with 0.1 M sodium hydroxide until a pink colour is obtained.
1 mL of 0.1 M sodium hydroxide is equivalent to 39.26 mg of C24H40O4.
IMPURITIES
Specified impurities: A, C.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.
Control of impurities in substances for pharmaceutical use): B, D, E, F, G, H, I.
A. 3α,7α-dihydroxy-5β-cholan-24-oic acid (chenodeoxycholic acid),
B. 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid (cholic acid),
C. 3α-hydroxy-5β-cholan-24-oic acid (lithocholic acid),
D. 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid (ursocholic acid),
E. 3α,12α-dihydroxy-5β-cholan-24-oic acid (deoxycholic acid),
F. 3α-hydroxy-7-oxo-5β-cholan-24-oic acid,
G. methyl 3α,7β-dihydroxy-5β-cholan-24-oate,
H. 3β,7β-dihydroxy-5β-cholan-24-oic acid,
I. 5β-cholane-3α,7β,24-triol.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description S24/25 - Avoid contact with skin and eyes.
S36 - Wear suitable protective clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
UN IDs UN1230 - class 3 - PG 2 - Methanol, solution
WGK Germany 2
RTECS FZ2000000
FLUKA BRAND F CODES 10
HS Code 2918190090
Ursodeoxycholic Acid (UDCA; Ursodiol) (CAS: 128-13-2) is a chemical agent of natural bile acid which is isolated from the bile of bear. It is the stereo-isomer of Chenodeoxycholic Acid. It has a similar litholysis effect, efficacy as Chenodeoxycholic Acid. However, it has a short course of treatment and a small dose. It is bound with taurine in the bile in vivo, and is a hydrophilic bile acids as well as a dissolving agent of cholesterol. It can reduce the secretion of cholesterol in the liver, lower the saturation content of cholesterol in bile, promote the secretion of bile acids, and increase the solubility of cholesterol in the bile so that cholesterol gallstones can be dissolved or prevented. Moreover, it can increase the secretion amount of bile, and have a choleretic effect by relaxing the bile duct mouth sphincter which smoothen the discharge of calculus. This product, however, cannot dissolve other types of gallstones. Ursodeoxycholic Acid is useful in the treatment of cholesterol stones, hyperlipidemia, bile secretion disorders, primary biliary cirrhosis, chronic hepatitis, bile reflux gastritis and prevention of liver allograft rejection and reaction. The calculus-dissolving effect of this product is slightly weaker than the CDCA.
Ursodeoxycholic Acid (UDCA; Ursodiol) (CAS: 128-13-2), a secondary bile acid, Gallstone dissolving drug. It is mainly used for cholesterol type gallstones that are not suitable for surgical treatment, especially for floating cholesterol stones with basically normal gallbladder function, stone diameter of less than 15mm, X-ray and non-calcification, which have a high cure rate. It also has a certain therapeutic effect on toxic hepatitis, cholecystitis, primary sclerosing cholangitis and primary cholestasis cirrhosis. Suitable for the prevention and treatment of cholesterol gallstones.
Ursodeoxycholic Acid is used primarily as a bulk drug to produce choleretic drugs. Since the 1970s, it has been used as an effective component in the treatment of cholesterol-type gallstones. In recent years, it has been found to reduce blood fat, lower blood sugar, antispasta, anticonvulsant, hemolysis and lipase promotion. It is mainly used for the treatment of biliary diseases. For the treatment of gallstones, cholestatic liver disease, fatty liver, various types of hepatitis, toxic liver disorders, cholecystitis, biliary and biliary dyspepsia, bile reflux gastritis.
Function of Ursodeoxycholic Acid (UDCA; Ursodiol) (CAS: 128-13-2)
1 Can increase the secretion of bile acid.
2 Reduce cholesterol in bile and cholesterol ester.
3 Is conducive to the cholesterol gradually dissolve gallstones.
4 Should not be used for surgical treatment of cholesterol stone, but cannot dissolve the bile pigment stone, mixing and opaque X line of stones.
Ursodeoxycholic Acid: Treatment of primary biliary cirrhosis;
Ursodeoxycholic Acid: Prevention of acute rejection in patients with liver transplantation;
Ursodeoxycholic Acid: Treatment of intrahepatic stones in Caroll's syndrome
(1) In combination with Chenodeoxycholic Acid, the effect of promoting cholesterol level and de-saturation in bile were more than single drugs. The effect is also greater than that of the sum of the two drugs.
(2) This product is not suitable taken together with cholestyramine or antacids containing aluminum hydroxide for not affecting the absorption.
(3) Oral contraceptives may affect the efficacy of the product.
Ursodeoxycholic Acid has a small side effects than Chenodeoxycholic Acid. It generally doesn’t cause diarrhea. Occasional occurrence of constipation, allergies, headaches, dizziness, pancreatitis, and tachycardia.
-
Chenodeoxycholic Acid (CDCA) CAS 474-25-9 Assay...
-
Cholic Acid CAS 81-25-4 Purity >98.0% (HPLC) Fa...
-
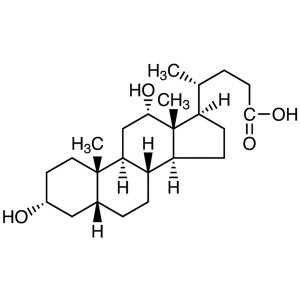
Deoxycholic Acid CAS 83-44-3 Purity >98.0% (T) ...
-
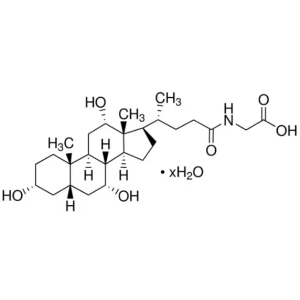
Glycocholic Acid Hydrate CAS 475-31-0 Assay 98....
-
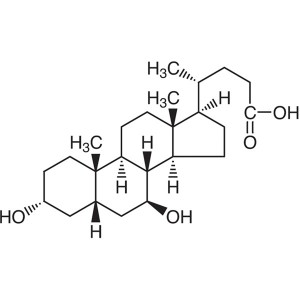
Ursodeoxycholic Acid (UDCA) CAS 128-13-2 Assay ...
-
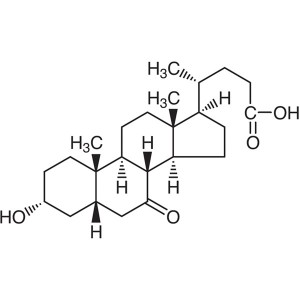
3α-Hydroxy-7-oxo-5β-Cholanic Acid CAS 4651-67-6...
-
Sodium Deoxycholate CAS 302-95-4 Assay 97.5~102.5%
-
Cholesterol CAS 57-88-5 High Quality
-
Hyodeoxycholic Acid (HDCA) CAS 83-49-8 Assay 99...
-
Tauroursodeoxycholic Acid Dihydrate CAS 14605-2...