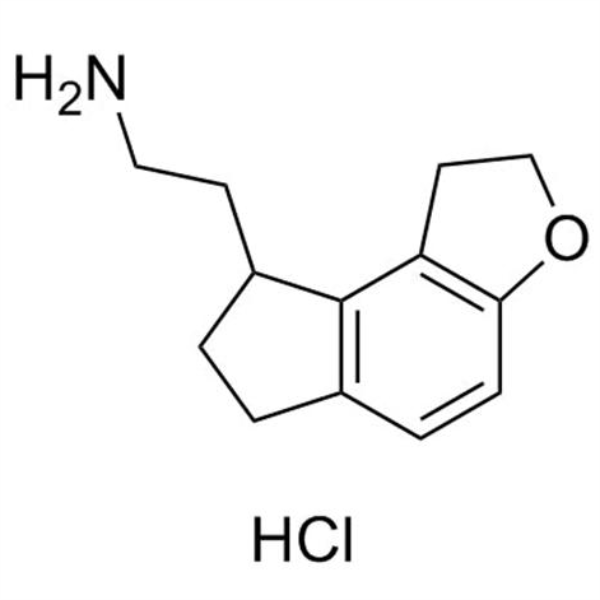
Ramelteon Intermediate 12 CAS 1053239-39-6 Purity >98.0% (HPLC) High Quality
Shanghai Ruifu Chemical is the leading supplier of 2-(2,6,7,8-Tetrahydro-1H-Indeno[5,4-b]furan-8-yl)ethanamine Hydrochloride (CAS: 1053239-39-6) with high quality, an intermediates of Ramelteon (CAS: 196597-26-9).
Ruifu Chemical has been supplying pharmaceutical intermediates and API (active pharmaceutical ingredient) more than 15 years, can provide worldwide delivery, competitive price, excellent service.
Purchase intermediates of Ramelteon, please e-mail: alvin@ruifuchem.com
| Chemical Name | 2-(2,6,7,8-Tetrahydro-1H-Indeno[5,4-b]furan-8-yl)ethanamine Hydrochloride |
| Synonyms | Ramelteon Intermediate 12; Ramelteon Stage-2 Impurity; 1,6,7,8-Tetrahydro-2H-Indeno[5,4-B]Furan-8-Ethanamine Hydrochloride; Ramelteon Impurity 7; Ramelteon Impurity 7 HCl |
| CAS Number | 1053239-39-6 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C13H18ClNO |
| Molecular Weight | 239.74 |
| Melting Point | 165.0~167.0℃ |
| Density | 1.058±0.06 g/cm3 |
| Shelf Life | 24 Months When Properly Stored |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Solid Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Loss on Drying | <1.00% |
| Single Impurity | <1.00% |
| Total Impurities | <2.00% |
| 1 H NMR Spectrum | Consistent With Structure |
| Test Standard | Enterprise Standard |
| Application | Intermediate of Ramelteon (CAS: 196597-26-9) |
Package: Bottle, aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-8℃) and well-ventilated warehouse away from incompatible substances. Prevent direct sunlight; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2-(2,6,7,8-Tetrahydro-1H-Indeno[5,4-b]furan-8-yl)ethanamine Hydrochloride (CAS: 1053239-39-6) is an intermediate of Ramelteon (CAS: 196597-26-9).
Unlike most treatments of insomnia that target the GABA (g-aminobutyric acid) receptor complex, Ramelteon is an agonist of the melatonin receptor. In particular, it has high selectivity for the MT1 and MT2 subtypes, which have been implicated in the maintenance of circadian rhythms, over the MT3 receptor responsible for other melatonin functions. Its lack of affinity for not only the GABA receptor complex but also neurotransmitter, dopaminerigic, opiate, and benzodiazepine receptors suggests an improved safety profile devoid of the abuse potential of the hypnotic drugs that target these receptors. As such, Ramelteon is not a scheduled drug. Primary metabolites include hydroxylation and oxidation to carbonyl species with secondary metabolites resulting from glucuronidation. Since CYP1A2 is the major isozyme involved in the hepatic metabolism of Ramelteon, it should not be taken in combination with strong CYP1A2 inhibitors, such as fluvoxamine. Co-administration with either ketoconazole (a CYP3A4 inhibitor) or fluconazole (a potent CYP2C9 inhibitor) resulted in significant increases in AUC and Cmax, but the extensive metabolism and highly variable plasma concentrations of Ramelteon precluded the need for dose modification. The package insert, however, cautions patients about co-administration with potent CYP3A4 and CYP2C9 inhibitors. Based on the result of the clinical trials, the recommended dose of Ramelteon is 8mg taken within 30 min of going to bed. In addition to the precaution of co-administration with CYP inhibitors, it should not be used in patients with severe hepatic impairment. The adverse events, observed in 5% of patients in clinical studies, were somnolence, dizziness, nausea, fatigue, headache, and insomnia.
-
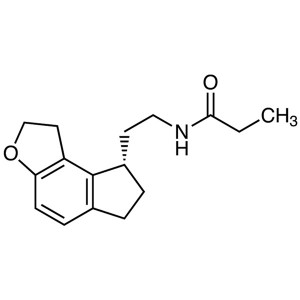
Ramelteon (TAK-375) CAS 196597-26-9 Purity >99....
-
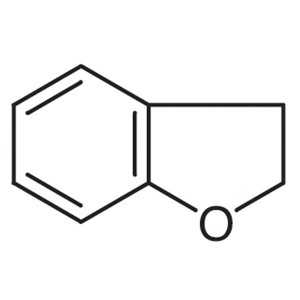
2,3-Dihydrobenzofuran CAS 496-16-2 Ramelteon In...
-
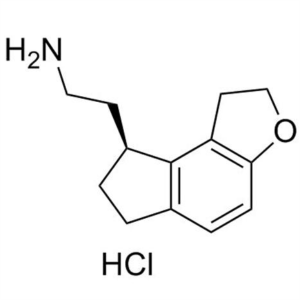
Despropionyl Ramelteon Hydrochloride CAS 196597...
-
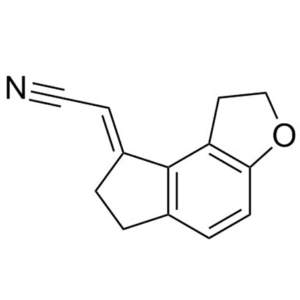
Ramelteon Acrylonitrile CAS 196597-79-2 Ramelte...
-
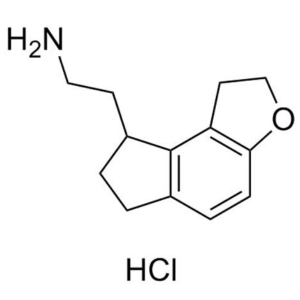
Ramelteon Intermediate 12 CAS 1053239-39-6 Puri...
-
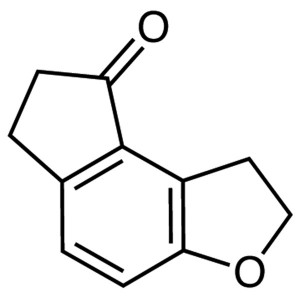
Ramelteon Intermediate CAS 196597-78-1 Purity >...