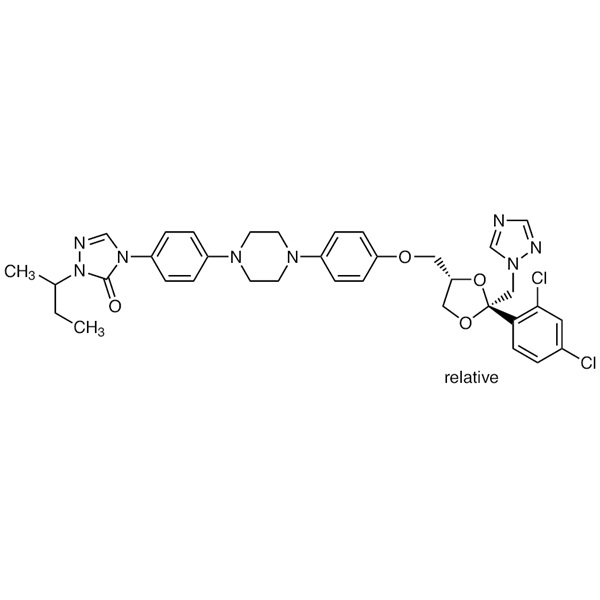
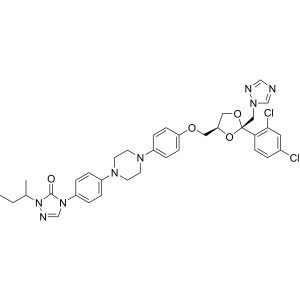
Itraconazole CAS 84625-61-6 Assay 98.5~101.5%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Itraconazole (CAS: 84625-61-6) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Itraconazole, Please contact: alvin@ruifuchem.com
| Chemical Name | Itraconazole |
| Synonyms | Sporanox, R51211, Oriconazole; (+/-)-4-[4-[4-[4-[[(2R,4S)-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl)-3H-1,2,4-triazol-3-one |
| Stock Status | In Stock |
| CAS Number | 84625-61-6 |
| Molecular Formula | C35H38Cl2N8O4 |
| Molecular Weight | 705.64 g/mol |
| Melting Point | 166.0~170.0℃ |
| Flash Point | >110℃(230°F) |
| Density | 1.27 g/cm3 |
| Sensitive | Heat Sensitive |
| Water Solubility | Insoluble in Water |
| Solubility | Solube in Chloroform at 50 mg/ml. Slightly Soluble in Ethanol or Methanol |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Category | API |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Almost White Powder; Odorless, Tasteless | Complies |
| Melting Point | 166.0 to 170.0℃ | 166.1~166.6℃ |
| Optical Rotation | -0.10° to +0.10° | Complies |
| Identification | (1) HPLC: The retention time of the major peak of the test solution corresponds to that of the standard solution | Complies |
| (2) Infrared absorption spectrum of the sample should be consistent with that of the reference standard spectrum | Complies | |
| Clarity and Color of Dichloromethane Solution | Add 10ml of Dichloromethane to dissolve, the solution should be clear and colorless; If it is turbid, it should not be more concentrated compared with No. 1 turbidity standard solution; If it is colored, no deeper color shall be compared with the orange-yellow or brown-red standard colorimetric solution No. 4 |
Complies |
| Loss on Drying | ≤0.50% (at 105℃ for 4 hours) | 0.06% |
| Residue on Ignition | ≤0.10% | 0.05% |
| Heavy Metals (Pb) | ≤20ppm | <20ppm |
| Any Specified Impurity | ≤0.50% | Complies |
| Total Impurities | ≤1.25% | Complies |
| Assay / Analysis Method | 98.5~101.5% (Calculated on the dried basis) | 99.5% |
| Conclusion | The product has been tested & complies with the given specifications | |
| Notes | Research Use only: Not intended for animal or human diagnostic or therapeutic use. | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in a tightly closed container. Store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Itraconazole contains not less than 98.5 percent and not more than 101.5 percent of C35H38Cl2N8O4, calculated on the dried basis.
Packaging and storage-Preserve in tight, light-resistant containers, and store at room temperature.
USP Reference standards <11>-
USP Itraconazole RS
USP Miconazole RS
Identification-
A: Infrared Absorption <197K>.
Angular rotation <781A>: between -0.10° and +0.10°, measured at 20°
Test solution: 100 mg per mL, in methylene chloride
Melting range <741>: between 166° and 170°
Loss on drying <731>-Dry about 1 g at 105° for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition <281>: not more than 0.1%, determined on 1.0 g.
Related compounds-
Solution A: 0.08 M tetrabutylammonium hydrogen sulfate.
Solution B: acetonitrile
Diluent-Prepare a mixture of methanol and tetrahydrofuran (1:1).
Standard solution-Dissolve an accurately weighed quantity of USP Itraconazole RS in Diluent, stepwise if necessar y, to obtain a solution having a known concentration of about 0.05 mg per mL.
Resolution solution-Dissolve suitable quantities of USP Itraconazole RS and USP Miconazole in RS in Diluent to obtain a solution having known concentrations of about 0.05 mg each per mL.
Test solution-Dissolve an accurately weighed quantity of Itraconazole in Diluent to obtain a solution having a known concentration of about 10 mg per mL.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 225-nm detector and a 4.6-mm × 10-cm column that contains 3- μm packing L1. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 30 °. The chromatograph is programmed as follows
Time Solution A Solution B
(minutes) (%) (%) Elution
0–20 80→50 20→50 linear gradient
20–25 50 50 isocratic
25–30 80 20 equilibration
[NOTE-Equilibrate the column for at least 30 minutes with acetonitrile at a flow rate of 1.5 mL per minute and then equilibrate at the initial eluent composition for at least 5 minutes.]
Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the resolution, R, between miconazole and itraconazole is not less than 2.0.
Procedure-Separately inject equal volumes (about 10 µL) of the Diluent, the Standard solution and the Test solution into the chromatograph and record the chromatograms. Calculate the percentage of each impurity in the portion of Itraconazole taken by the formula:
100(CS / CU)(rU / rS)
in which CS is the concentration, in mg per mL, of Itraconazole in the Standard solution; CU is the concentration, in mg per mL, of Itraconazole in the Test solution; rU is the peak area for each impurity in the Test solution; and rS is the peak area for Itraconazole in the Standard solution: not more than 0.5% of any specified impurity, as shown in Table 1, is found; and not more than 1.25% of total impurities is found. Disregard any peak observed in the Diluent and any peak less than 0.05%.
Table 1
Common Name Limit (%)
4-Methoxy derivative1 0.5
4-Triazolyl isomer2 0.5
Propyl analog3 0.5
Isopropyl analog4 0.5
Epimer5 0.5
n-Butyl isomer6 0.5
Didioxolanyl analog7 0.5
1 2-sec-Butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one.
2 4-(4-{4-[4-({(2RS,4SR)-2-[(4H-1,2,4-Triazol-4-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-sec-butyl-2H-1,2,4-triazol-3(4H)-one.
3 4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-propyl-2H-1,2,4-triazol-3(4H)-one.
4 4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-isopropyl-2H-1,2,4-triazol-3(4H)-one.
5 4-(4-{4-[4-({(2 chelating agents. RS,4RS)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-sec-butyl-2H-1,2,4-triazol-3(4H)-one.
6 4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-butyl-2H-1,2,4-triazol-3(4H)-one.
7 4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-({(2RS,4SR)-2-[(1H-1,2,4-triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxo-lan-4-yl}methyl)-2H-1,2,4-triazol-3(4H)-one.
Assay-
Diluent-Prepare a mixture of methyl ethyl ketone and glacial acetic acid (7:1).
Procedure-Dissolve about 0.3 g of Itraconazole, accurately weighed, in 70 ml of Diluent. Titrate with 0.1 M per chloric acid, determining the endpoint potentiometrically at the second inflection point. One mL of 0.1 M per chloric acid is equivalent to 35.3 mg of C 35H38Cl2N8O4.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R36/37/38 - Irritating to eyes, respiratory system and skin.
R36/38 - Irritating to eyes and skin.
R22 - Harmful if swallowed
R39/23/24/25 -
R23/24/25 - Toxic by inhalation, in contact with skin and if swallowed.
R11 - Highly Flammable
Safety Description
S22 - Do not breathe dust.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37 - Wear suitable protective clothing and gloves.
S16 - Keep away from sources of ignition.
UN IDs UN 3286 8(6.1)(3) / PGII
WGK Germany 3
RTECS XZ5481000
Toxicity LD50 (14 day) in mice, rats, dogs (mg/kg): >320, >320, >200 orally (Van Cauteren)
Itraconazole (CAS: 84625-61-6) is a synthetic triazole derivative. It is a synthetic broad-spectrum antifungal drug. Its antibacterial spectrum and antibacterial mechanism are similar to clotrimazole, but it has strong antibacterial activity against Aspergillus. The permeability of fungal cell membrane exerts antibacterial activity, and it has antibacterial activity against pathogens of superficial and deep fungal infections. Its antibacterial spectrum is broader and stronger than ketoconazole. It can inhibit the synthesis of fungal cell membrane ergosterol, thereby exerting antifungal effect. This product is effective against dermatophytes (Trichophyton, Microsporum, Epidermophyton flocculus), yeasts (Cryptococcus neoformans, Saccharomyces sp., Candida (including Candida albicans, Candida glabrata and Candida krusei)], Aspergillus, Histoplasma, Paracoccosis Brazil, Sporothrix schenckii, Color fungi, Cladosporium, Blastomyces dermatitis, and various other yeasts and fungi Has an inhibitory effect. But itraconazole cannot inhibit the growth of Rhizopus and Mucor.
Function:
1) Itraconazole has a broader spectrum of activity than fluconazole (but not as broad as voriconazole or posaconazole). In particular, it is active against Aspergillus which fluconazole is not.
2) It is also prescribed for systemic infections, such as aspergillosis, candidiasis, and cryptococcosis
3) Itraconazole has also recently been explored as an anticancer agent for patients with basal cell carcinoma.
Itraconazole is indicated for the treatment of the following diseases:
1. For systemic fungal infections, such as aspergillosis, candidiasis, cryptococcosis (including cryptococcal meningitis), histoplasmosis, sporotrichosis, Brazilian paracoccosis, blastomycosis And many other rare systemic or tropical fungal diseases.
2. Used for oral cavity, pharynx (foreign data), esophagus (foreign data), vulvovaginal Candida infection, fungal conjunctivitis, fungal keratitis.
3. Used for superficial fungal infections, such as tinea hands, tinea corporis, tinea cruris, tinea versicolor, etc.
4. For onychomycosis caused by dermatophytes and (or) yeasts.
-
Itraconazole CAS 84625-61-6 Assay 98.5~101.5%
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Baloxavir Marboxil CAS 1985606-14-1 API Factory...
-
Betamethasone CAS 378-44-9 Purity 97.0%~103.0% ...
-
Bicalutamide CAS 90357-06-5 API Factory High Qu...
-
Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-
Brexpiprazole CAS 913611-97-9 Purity >99.0% (HP...
-
Candesartan Cilexetil CAS 145040-37-5 Purity >9...
-
Captopril CAS 62571-86-2 API Factory USP High Q...
-
Cefotaxime Sodium Salt CAS 64485-93-4 Assay ≥91...
-
Cholestyramine CAS 11041-12-6 USP API Factory H...
-
Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...
-
Cisatracurium Besylate CAS 96946-42-8 Assay 95....
-
Citicoline Sodium Salt Hydrate CAS 33818-15-4 A...
-
Crizotinib CAS 877399-52-5 Assay ≥99.0% API Fac...
-
Daptomycin CAS 103060-53-3 Purity ≥95.0% API Fa...