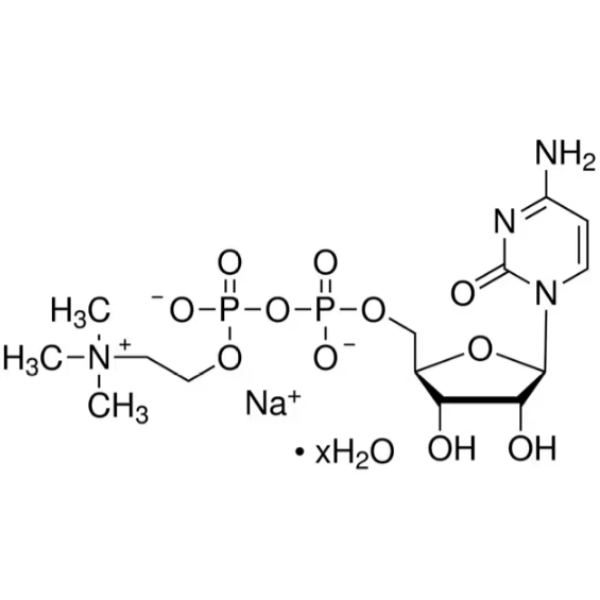
Citicoline Sodium Salt Hydrate CAS 33818-15-4 Assay ≥98.0% High Purity
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Citicoline Sodium (CAS: 33818-15-4) with high quality, commercial production. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Citicoline Sodium, Please contact: alvin@ruifuchem.com
| Chemical Name | Citicoline Sodium Salt Hydrate |
| Synonyms | Citicoline Sodium Salt; CDPC; CDP-Choline Sodium Salt; Cytidine 5'-Diphosphocholine Sodium Salt Dihydrate |
| CAS Number | 33818-15-4 |
| Related CAS | 987-78-0 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C14H25N4NaO11P2·xH2O |
| Molecular Weight | 510.31 (Anhydrous Basis) |
| Melting Point | 259.0~268.0℃ (dec.) |
| Water Solubility | Soluble in Water (100 mg/ml) |
| Condition to Avoid | Hygroscopic, Heat Sensitive |
| Sensitive | Easily Absorbing Moisture |
| COA & MSDS | Available |
| Shelf Life | Limited Shelf Life, Expiry Date On The Label |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | White Crystal or Crystalline Powder, Odorless | White Crystalline Powder, Odorless |
| Solubility | Freely Soluble in Water. Insoluble in Ethanol, Acetone, Chloroform | Conforms |
| Identification | ||
| The color of the solution reaction was positive reaction | Conforms | |
| The retention time of major peak of the sample solution should correspond to that of reference standard. | Conforms | |
| The infrared absorption spectrum is concordant with the reference spectrum | Conforms | |
| The aquous solution yields the reaction characteristic of sodium salts | Conforms | |
| pH | 6.0~7.5 | 6.9 |
| Clarity and Color of Solution | Should be Clear and Colorless | Conforms |
| Chlorides | ≤0.05% | Conforms |
| Ammonium Salt | ≤0.05% | Conforms |
| Iron Salt | ≤0.01% | Conforms |
| Phosphate | ≤0.10% | Conforms |
| Loss on Drying | ≤6.00% | 1.0% |
| Heavy Metals | ≤0.0005% | Conforms |
| Arsenic Salt | ≤0.0001% | Conforms |
| Microbial Limit Test | ||
| Bacterial Endotoxins | ≤0.30 EU/mg | Conforms |
| Total Bacterial Count | ≤1000cfu/g | <10cfu/g |
| Yeast & Molds | ≤100cfu/g | <10cfu/g |
| E. Coli | Not Detected | Not Detected |
| Related Substanccs |
||
| 5'-CMP | ≤0.30% | 0.022% |
| Other Simple Impurity | ≤0.20% | 0.002% |
| Other Total Impurities | ≤0.70% | 0.004% |
| Residual Solvents | ||
| Methanol | ≤0.30% | Not Detected |
| Ethanol | ≤0.50% | Not Detected |
| Acetone | ≤0.50% | Not Detected |
| Assay | ≥98.0% Citicoline Sodium, Calculated on the dried basis | 100.5% |
| Conclusion | Inspection accords with the standard of CP2010 (None-Sterile APIS) | |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture. Incompatible with oxidizing agents.

| Safety Description | 24/25 - Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| FLUKA BRAND F CODES | 3-10-21 |
| HS Code | 2923100000 |
Citicoline is a nucleic acid derivatives, Geiger found that citicoline can restore brain injury in the animal experiments in 1956. Kennedy study confirmed that citicoline can make brain injury recovery in 1957. It was registered in China in 1988, and is currently the best-selling drug among clinical brain diseases. It plays an important role in the synthesis of lecithin, by promoting the synthesis of lecithin and improving brain function. Experiments show that citicoline can enhance the levels of norepinephrine and dopamine in the central nervous system, thereby it can treat cerebrovascular disease, traumatic brain injury and cognitive impairment caused by various reasons, and there are no obvious side effects.
Citicoline sodium can enhance the function of brain stem reticular formation, especially the ascending reticular activating system associated with human consciousness; enhance the function of the pyramidal system; inhibit the function of the external system of the cone, and promotes the recovery of the function of the system. For the treatment of sequelae of traumatic brain injury and cerebral vascular accident caused by the nervous system, it can also be used in the treatment of Parkinson's disease, senile dementia has a certain effect; for the treatment of cardiovascular and cerebrovascular diseases; it also have a certain effect for anti-aging, improving learning and memory.
33818-15-4 - Standard
This product is choline cytidine diphosphate monosodium salt, calculated as dry product, containing citicoline sodium (C14H25N4NaO11P2) not less than 98.0%.
33818-15-4 - Trait
This product is white crystal or crystalline powder; Odorless.
This product is soluble in water, insoluble in ethanol and acetone.
33818-15-4 - Differential diagnosis
Take about 1 mg of this product, add 3ml of dilute hydrochloric acid and 1ml of bromine test solution, heat it in a water bath for 30 minutes, put it in a ventilated place, and add 3 after bromine is removed, 5-dihydroxytoluene ethanol solution (1-10 ) 0.2ml, and then human ferrous ammonium sulfate hydrochloric acid solution (l-l000 )3ml, water bath heating 20 minutes, the solution is green.
In the chromatogram recorded under the content determination item, the retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference solution.
The infrared absorption spectrum of this product should be consistent with that of the control (Spectrum set 1096).
The product of the aqueous solution of sodium salt identification (1) of the reaction (General 0301).
33818-15-4 - Exam
pH
Take 0.5g of this product, Add 10ml of water to dissolve, and measure according to law (General rule 0631). The pH value should be 6.0~7.5.
Clarity and color of solution
Take 1.0g of this product, add water to dissolve 8ml, and check according to law (General rule 0901 first method and general rule 0902 first method), the solution should be clear and colorless.
Chloride
Take 0.10g of this product and check it according to law (General rule 0801). Compared with the reference solution made of 0.05% of standard gasification sodium solution, it should not be more concentrated ().
Ammonium salt
take 0.20g of this product and check it according to law (General rule 0808). Compared with the reference solution made of 0.05% of standard ammonium chloride solution, it should not be more concentrated ().
Iron Salt
Take 0.20g of this product and check it according to law (General rule 0807). Compared with the control solution made of 2.0 ml of standard iron solution, it should not be deeper (0.01%).
Phosphate
Take 0.10g of this product, Add 10ml of water to dissolve, add 1 ml of ammonium molybdate solution (take lg of ammonium molybdate and dissolve 40ml of 0.5mol/L sulfuric acid solution), l-amino -2-naphthol -4-sulfonic acid test solution 0.5, placed for 5 minutes; With standard phosphorus solution (precision weighing 105g of potassium dihydrogen phosphate dried to constant weight at 0.286℃, put it in a 1000ml measuring flask, add water to dissolve and dilute to scale, shake well, take 10ml immediately before use, put it in a 100ml measuring flask, dilute it to scale with water, shake well. Each 1 ml is equivalent to 20ug of PO4) 0.1% compared with the control solution prepared by the same method, the color should not be darker ().
Related substances
Take this product, precision weighing, adding water to dissolve and quantitatively dilute to make a solution containing 2.5mg per lml as a test solution; Take lml for precision measurement and put it in a 500ml measuring flask, dilute to the scale with water, shake well, as a control solution; Take an appropriate amount of the 5 '-cytidine acid reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 7.5ug per 1 ml, as the chromatographic conditions under the content determination item of the reference solution, 10ul of each of the test solution, the control solution and the 5-cytidine acid reference solution are accurately measured and injected into the liquid chromatograph respectively, the chromatogram was recorded to 2.5 times the retention time of the main peak. If there are impurity peaks in the chromatogram of the test solution, the peak area shall be calculated according to the external standard method, and the content of 5-cytidine acid shall not exceed 0.3%; The peak area of other individual impurities shall not be greater than the main peak area of the control solution (0.2%) , the sum of the peak areas of other impurities shall not be greater than 3.5 times (0.7%) of the main peak area of the control solution.
Residual solvent
Accurately weigh an appropriate amount of N-propanol and dilute it with water to make a solution containing 500ug per 1 ml as an internal standard solution; Accurately weigh an appropriate amount of methanol, ethanol and acetone and place it in the same measuring flask, prepare solutions containing 450UG, 750ug and 750ug in each lml by quantitative dilution with water as the reference stock solution; Take 5ml of the reference stock solution and 5ml of the internal standard solution in the same 50ml measuring flask, dilute to scale with water, shake, as a reference solution; Weigh 0.75g of this product accurately, put it in a 50ml measuring flask, add 5ml of internal standard solution to dissolve and dilute to scale with water, shake, as a test solution. Take 5.0ml of each of the reference solution and the test solution respectively, place them in the top empty bottle respectively, seal them, and measure them according to the method of residual solvent determination (General rule 0861 first method). The capillary column used as the stationary liquid was a chromatographic column; The column temperature was 60℃; The injection port temperature was 200℃; The detector temperature was 250℃; The headspace bottle equilibrium temperature was 80℃, and the equilibrium time was 45 minutes. Take the reference solution into the headspace, the separation degree between the peaks of each component shall meet the requirements. The test solution and the reference solution were respectively injected in headspace, Record the chromatogram, according to the internal standard method to calculate the peak area, methanol, ethanol and acetone residues should be in accordance with the provisions.
Loss on drying
take this product, with phosphorus pentoxide as desiccant, drying at 100℃ under reduced pressure for 5 hours, loss of weight shall not exceed 6.0% (General rule 0831).
Heavy metals
take this product 2.0g, inspection according to law (General Principles 0821 The first law), containing heavy metals shall not exceed 5 parts per million.
Arsenic salt
Take this product 2.0g, inspection according to law (General Principles 0822 The first law), should comply with the provisions (0.0001%).
Bacterial endotoxin
Take this product, check according to law (General rule 1143), each 1 mg citicoline sodium containing endotoxin amount should be less than 0.30EU.
Sterile
take this product, dissolve it with appropriate solvent, and treat it by membrane filtration method. Check it according to law (General rule 1101). (For aseptic dispensing)
33818-15-4 - Content determination
Measured by high performance liquid chromatography (General 0512).
Chromatographic conditions and system suitability test
Silica gel bonded with eighteen alkyl silane as filler; Phosphate buffer [0.1 mol/L potassium dihydrogen phosphate solution and tetrabutylammonium solution (O.Olmol/L tetrabutylammonium hydroxide solution was adjusted to pH 4.5 with phosphoric acid. The mobile phase consisted of equal mixing]-methanol (95:5). The detection wavelength was 276nm. Take an appropriate amount of the 5 '-cytidine acid reference, add water to dissolve and dilute to make a solution containing about 0.25mg per 1 ml. Take the appropriate amount, mix it with the citicoline sodium reference solution in the same amount, shake well, and inject 20ul into the liquid chromatograph, adjust the chromatographic system, citicoline peak and cytidine acid peak separation should meet the requirements.
Assay
Take an appropriate amount of this product, weigh it accurately, add water to dissolve and dilute it to prepare a solution containing about 0.25mg per 1 ml as a test solution, A 10ul injection liquid chromatograph was used for precise measurement, and the chromatogram was recorded. An appropriate amount of citicoline sodium reference substance was taken and determined by the same method, and the peak area was calculated according to the external standard method.
-
Citicoline Sodium Salt Hydrate CAS 33818-15-4 A...
-
Citicoline CAS 987-78-0 CDP-Choline Purity ≥99....
-
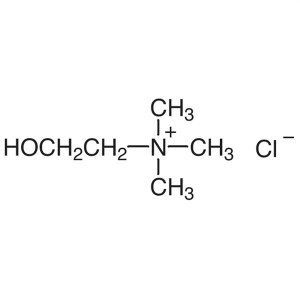
Choline Chloride CAS 67-48-1 Assay 99.0~100.5% ...
-
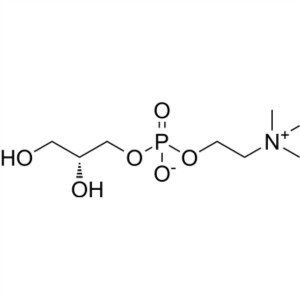
Choline Glycerophosphate CAS 28319-77-9 Assay 9...
-
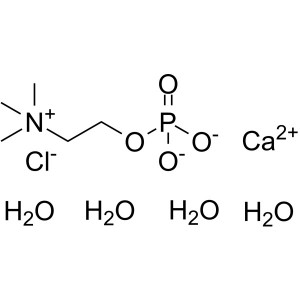
Phosphocholine Chloride Calcium Salt Tetrahydra...
-
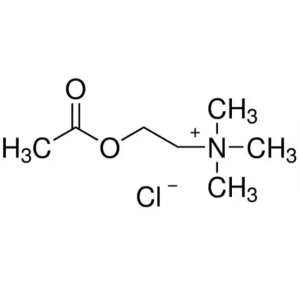
Acetylcholine Chloride CAS 60-31-1 Assay 98.0~1...
-
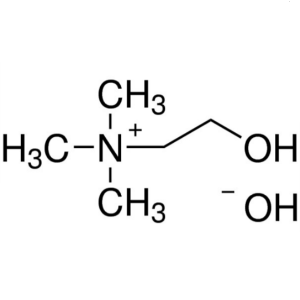
Choline Hydroxide Solution CAS 123-41-1 44 wt. ...