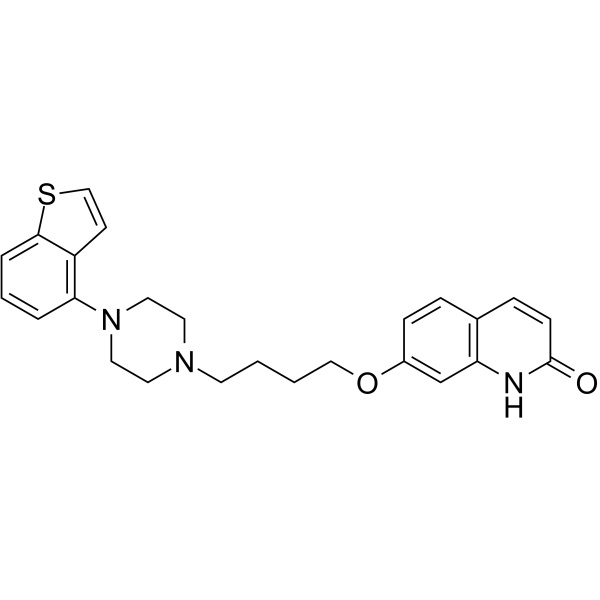
Brexpiprazole CAS 913611-97-9 Purity >99.0% (HPLC) API Factory
Ruifu Chemical Supply Brexpiprazole Intermediates
Brexpiprazole CAS 913611-97-9
7-Hydroxyquinolinone CAS 70500-72-0
4-Bromobenzo[b]thiophene CAS 5118-13-8
1-(1-Benzothiophen-4-yl)piperazine Hydrochloride CAS 913614-18-3
7-(4-Chlorobutoxy)quinolin-2(1H)-one CAS 913613-82-8
4-Chlorobenzo[b]thiophene CAS 66490-33-3
| Chemical Name | Brexpiprazole |
| Synonyms | OPC-34712; 7-(4-(4-(Benzo[b]thiophen-4-yl)piperazin-1-yl)butoxy)quinolin-2(1H)-one |
| CAS Number | 913611-97-9 |
| CAT Number | RF-PI1982 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C25H27N3O2S |
| Molecular Weight | 433.57 |
| Density | 1.245±0.060 g/cm3 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Crystalline Powder |
| Identification | By IR |
| RT | Under chirality should complies |
| Chloride | It gives a reaction of chloride |
| Water | 3.0%~4.5% |
| Melting Point | 198.0~202.0℃ |
| Residue on Ignition | <0.20% |
| Heavy Metals | ≤10ppm |
| Related Substances | |
| Individual Impurity | <0.50% |
| Total Impurities | <1.00% |
| E.E | >99.0% |
| Purity / Analysis Method | >99.0% (HPLC, on dry basis) |
| Test Standard | Enterprise Standard |
| Usage | API |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Brexpiprazole (CAS: 913611-97-9) is a drug candidate useful in treatment and prevention of mental disorders including CNS disorders. Brexpiprazole is a novel antipsychotic drug which serves as a serotonin ® dopamine activity modulator and has demonstrated efficacy as an adjunctive treatment in patients with major depressive disorder (MDD). Brexpiprazole exhibits a unique pharmacological profile, acting as a partial agonist of serotonin 5-HT1A and dopamine D2 receptors and as a full antagonist of 5-HT2A and noradrenaline α1B/2C receptors, with similar subnanomolar binding affinity. Brexpiprazole was developed by Otsuka and Lundbeck, was approved in 2015 by the FDA for the treatment of schizophrenia and as an adjunctive treatment for depression. Brexpiprazole is widely considered to be a successor to Otsuka’s antipsychotic drug aripiprazole (trade name Abilify) whose patent expired in August 2014.
-
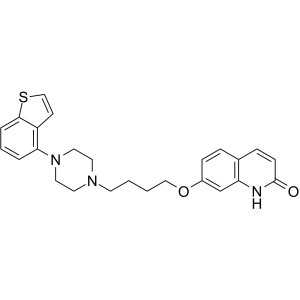
Brexpiprazole CAS 913611-97-9 Purity >99.0% (HP...
-
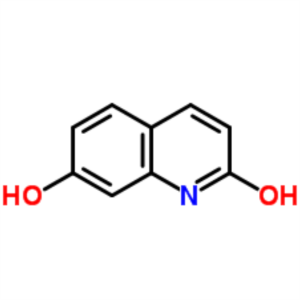
7-Hydroxyquinolinone CAS 70500-72-0 Purity >98....
-
![4-Bromobenzo[b]thiophene CAS 5118-13-8 Purity >97.0% (GC) Brexpiprazole Intermediate Manufacturer](https://www.ruifuchem.com/uploads/4-Bromobenzothiophene-CAS-5118-13-8-Purity-97.0-GC-Manufacturer-300x300.png)
4-Bromobenzo[b]thiophene CAS 5118-13-8 Purity >...
-
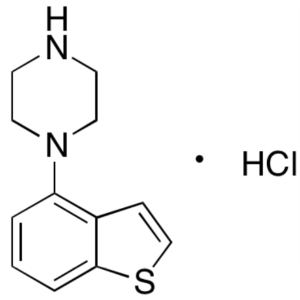
1-(1-Benzothiophen-4-yl)piperazine Hydrochlorid...
-
![4-Chlorobenzo[b]thiophene CAS 66490-33-3 Purity >98.0% (GC) Brexpiprazole Intermediate Factory](https://www.ruifuchem.com/uploads/4-Chlorobenzobthiophene-CAS-66490-33-3-Purity-98.0-GC-Brexpiprazole-Intermediate-Factory-300x300.png)
4-Chlorobenzo[b]thiophene CAS 66490-33-3 Purity...
-
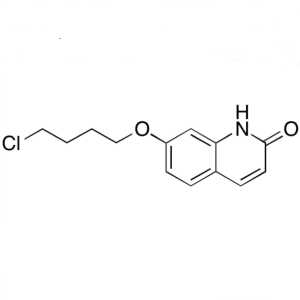
7-(4-Chlorobutoxy)quinolin-2(1H)-one CAS 913613...
-
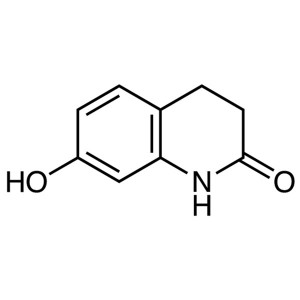
7-Hydroxy-3,4-Dihydro-2(1H)-Quinolinone CAS 222...