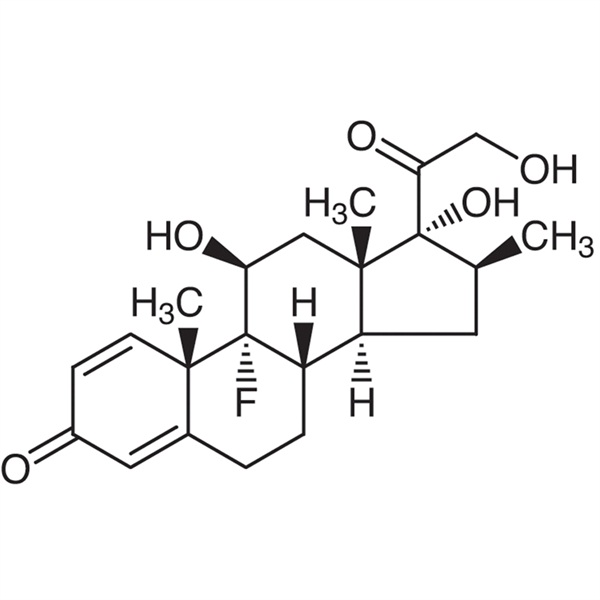
Betamethasone CAS 378-44-9 Purity 97.0%~103.0% API Factory High Purity
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Betamethasone (CAS: 378-44-9 ) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Betamethasone, Please contact: alvin@ruifuchem.com
| Chemical Name | Betamethasone |
| Synonyms | Betamethasone Base; 9alpha-Fluoro-16beta-Methylprednisolone; 9-Fluoro-11,17,21-Trihydroxy-16-Methylpregna-1,4-Diene-3,20-Dione; (11beta,16alpha)-9-Fluoro-11,17,21-Trihydroxy-16-Methylpregna-1,4-Diene-3,20-Dione |
| CAS Number | 378-44-9 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C22H29FO5 |
| Molecular Weight | 392.47 |
| Melting Point | 235.0~237.0℃ |
| Shipping Condition | Under Ambient Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystalline Powder |
| Identification A | Infrared Absorption |
| Identification B | Thin-Layer Chromatographic Identification Test |
| Specific Optical Rotation | +118.0° to +126.0° (Calculated on the Dried Basis) |
| Individual Impurity | ≤1.00% |
| Total Impurities | ≤2.00% |
| Residual Solvents Methanol | ≤3000ppm |
| Residual Solvents Chloroform | ≤60ppm |
| Loss on Drying | ≤0.50% (Dry it at 105℃, 3 hours) |
| Residue on Ignition | ≤0.20% |
| Purity / Analysis Method | 97.0%~103.0% of C22H29FO5 Calculated on the Dried Basis |
| Test Standard | Chinese Pharmacopoeia (CP); EP10.0 and USP 42 |
| Packaging and Storage | Preserve in tight containers. Store between 2℃ and 30℃ . |
| Application | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~30℃) and well-ventilated warehouse away from incompatible substances. Stay away from strong light and heat, moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Risk Codes R40 - Limited evidence of a carcinogenic effect
R48/20/21 -
R61 - May cause harm to the unborn child
Safety Description S22 - Do not breathe dust.
S36 - Wear suitable protective clothing.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S53 - Avoid exposure - obtain special instructions before use.
WGK Germany 2
RTECS TU4000000
HS Code 2937229000
Toxicity LD50 oral in mouse: > 4500mg/kg
Betamethasone (CAS: 378-44-9), belongs to adrenal corticosteroids, it is a isomer of dexamethasone , and the role of betamethasone is similiar to prednisolone and dexamethasone , it has anti-inflammatory, anti-rheumatic, anti-allergic and suppression of the immune and other pharmacological effects, its anti-inflammatory effect is stronger than dexamethasone, triamcinolone, hydrocortisone etc. , it can reduce and prevent tissue response to inflammation and eliminate heat, redness and swelling caused by local non-infectious inflammation, thereby reducing the performance of inflammation, anti-inflammatory effect of this product 0.3mg is equal to dexamethasone 0.75mg, prednisone 5mg or 25mg cortisone . Betamethasone sodium retention effect is a hundred times more than hydrocortisone, in primary adrenal hypofunction, it can be used together with glucocorticoid for replacement therapy ,and it is used for preventing or inhibiting cell-mediated immune response, delaying allergic reactions and reducing the primary immune response expansion ,it is used for low renin and low aldosterone syndrome and autonomic neuropathy induced orthostatic hypotension. Currently betamethasone is also used for the treatment of active rheumatoid arthritis, rheumatoid arthritis, lupus, severe bronchial asthma, severe dermatitis, acute leukemia, atopic dermatitis, eczema, neurodermatitis, seborrheic dermatitis, and pruritus and comprehensive treatment of certain infections. The product is contraindicated in severe psychiatric history, active duodenal ulcer, recent gastrointestinal anastomosis, heavier osteoporosis, overt diabetes, severe hypertension, virus , bacterial, fungal infections which are failed to control by the use of antimicrobial agents , thrombophlebitis, skin infections, such as impetigo, tinea, jock itch and so on.
Betamethasone
C22 H29FO5 392.46
Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11,16)-.
9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione [378-44-9].
Betamethasone contains not less than 97.0 percent and not more than 103.0 percent of C22H29FO5, calculated on the dried basis.
Packaging and storage-Preserve in tight containers. Store between 2℃ and 30℃.
USP Reference standards <11>-
USP Betamethasone RS
Identification-
A: Infrared Absorption <197M>.
B: Thin-Layer Chromatographic Identification Test <201>-
Test solution-Prepare a solution of Betamethasone in dehydrated alcohol containing 0.5 mg per mL.
Developing solvent system: a mixture of chloroform and diethylamine (2:1).
Procedure-Proceed as directed in the chapter, except to locate the spots by lightly spraying with dilute sulfuric acid (1 in 2) and heating on a hot plate or under a lamp until spots appear.
Specific rotation <781S>: between +118 and +126, calculated on the dried basis.
Test solution: 5 mg per mL, in methanol.
Loss on drying 731-Dry it at 105 for 3 hours: it loses not more than 1.0% of its weight.
Residue on ignition 281: not more than 0.2%, a platinum crucible being used.
Ordinary impurities 466-
Test solution: methanol.
Standard solution: methanol.
Application volume: 10 µL.
Eluant: a mixture of toluene, acetone, methyl ethyl ketone, and formic acid (55:20:20:5), in a nonequilibrated chamber.
Visualization: 5.
Assay-
Mobile phase-Prepare a filtered and degassed mixture of water and acetonitrile (63:37). Make adjustments if necessary (see System Suitability under Chromatography 621).
Internal standard solution-Prepare a solution of propylparaben in alcohol having a known concentration of about 0.25 mg per mL.
Standard preparation-Dissolve an accurately weighed quantity of USP Betamethasone RS in alcohol to obtain a solution having a known concentration of about 0.2 mg per mL. Transfer 10.0 mL of this solution to a suitable vial, and add 10.0 mL of Internal standard solution, to obtain a Standard preparation having known concentrations of about 0.1 mg of betamethasone and about 0.125 mg of propylparaben per mL.
Assay preparation-Using about 80 mg of Betamethasone, accurately weighed, prepare as directed for Standard preparation.
Chromatographic system (see Chromatography 621)-The liquid chromatograph is equipped with a 240-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 1.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 1.0 for betamethasone and 1.4 for propylparaben; the resolution, R, between betamethasone and propylparaben is not less than 3.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C22H29FO5 in the portion of Betamethasone taken by the formula:
800C(RU / RS)
in which C is the concentration, in mg per mL, of USP Betamethasone RS in the Standard preparation; and RU and RS are the peak height ratios of the betamethasone peak and the internal standard peak obtained from the Assay preparation and the Standard preparation, respectively.
-
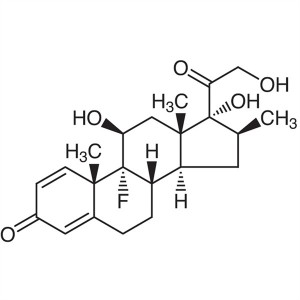
Betamethasone CAS 378-44-9 Purity 97.0%~103.0% ...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Abacavir CAS 136470-78-5 Assay 99.0%~101.0% API...
-
Abacavir Sulfate CAS 188062-50-2 Assay 98.0%~10...
-
Acotiamide Hydrochloride Trihydrate CAS 773092-...
-
Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPL...
-
CAS 665-66-7 Assay 98.5%~101.5% API
-
Anastrozole CAS 120511-73-1 API Factory High Qu...
-
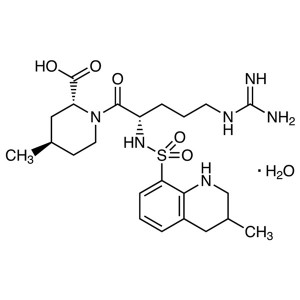
Argatroban Monohydrate CAS 141396-28-3 Purity ≥...
-
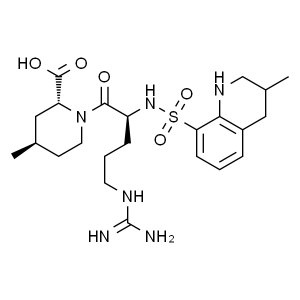
Argatroban CAS 74863-84-6 API Factory High Puri...
-
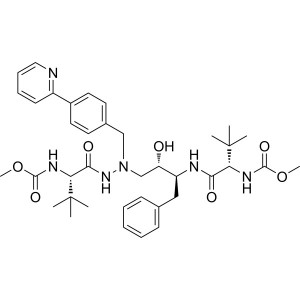
Atazanavir CAS 198904-31-3 Purity ≥99.0% API Fa...
-
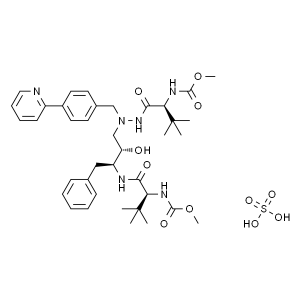
Atazanavir Sulfate CAS 229975-97-7 Purity ≥99.0...
-
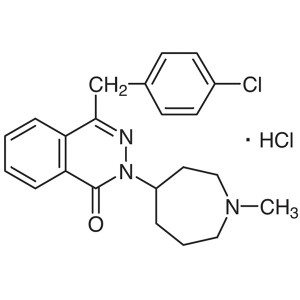
Azelastine Hydrochloride CAS 79307-93-0 Assay 9...
-
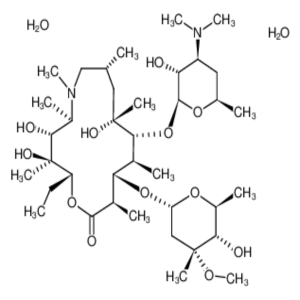
Azithromycin Dihydrate CAS 117772-70-0 Assay 94...