Crizotinib CAS 877399-52-5 Assay ≥99.0% API Factory High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Crizotinib (CAS: 877399-52-5) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Crizotinib, Please contact: alvin@ruifuchem.com
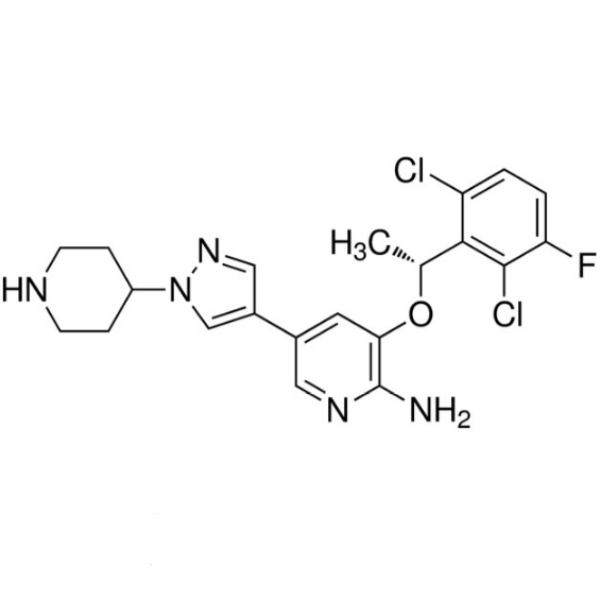
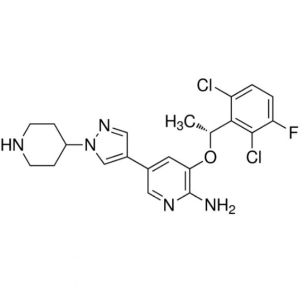
| Chemical Name | Crizotinib |
| Synonyms | Xalkori; PF-02341066; Crozotinib; Crizotinib Xalkori; 3-[1-(2,6-Dichloro-3-Fluoro-phenyl)-ethoxy]-5-(1-Piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine; (R)-3-[1-(2,6-Dichloro-3-Fluorophenyl)ethoxy]-5-(1-Piperidin-4-yl-1H- pyrazol-4-yl)pyridin-2-ylamine |
| CAS Number | 877399-52-5 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C21H22Cl2FN5O |
| Molecular Weight | 450.34 |
| Melting Point | 192℃ |
| Density | 1.47±0.10 g/cm3 |
| Storage Temperature | Room Temperature |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification | By IR, HPLC |
| Clarity of Solution | Conform to Standard |
| Loss on Drying | ≤1.00% |
| Residue on Ignition | ≤0.50% |
| Related Impurities | (by HPLC) |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.00% |
| Heavy Metals | ≤20ppm |
| Assay | ≥99.0% |
| Residual Solvents | Meet the Specification |
| Shelf Life | 24 Months |
| Test Standard | Enterprise Standard |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Safety Description 24/25 - Avoid contact with skin and eyes.
UN IDs UN 3077 9 / PGIII
WGK Germany 3
HS Code 2933990099
Hazard Class IRRITANT
Crizotinib (CAS 877399-52-5), (Crizotinib, Xalkori R ), is a potent and selective ATP competitive small molecule inhibitor of ALK and c-Met. In August 2011, the United States FDA approved Crizotinib for the treatment of anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC). Crizotinib is a dual ATP competitive inhibitor of tyrosine kinases c-MET (Mesenchymal-Epithelial Transition Factor) kinase (cellular IC50=8 nM) and ALK (cellular IC50=20 nM), both of which are important targets for cancer chemotherapy. When crizotinib was tested for selectivity versus other kinases it was found to have enzyme IC50's within 100-fold multiples of c-MET for 13 of the 120 kinases tested. In cellular assays, crizotinib was found to inhibit RON (recepteur d’origine nantais) kinase with a 10-fold selectivity window over c-MET.
Crizotinib (PF-02341066) Crizotinib is a potent c-Met and ALK inhibitor, the IC50 values in the cell assay were 11 nM and 24 nM, respectively. It is also a potent inhibitor of ROS1 with a Ki value of less than 0.025 nM. Crizotinib can induce autophagy in a variety of lung cancer cell lines by inhibiting the STAT3 pathway.
Crizotinib is a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. In August 2011, the United States FDA approved crizotinib for the treatment of anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC).
Crizotinib (Xalkori(R), Pfizer), approved in 2011, was the first approved inhibitor targeting anaplastic lymphoma kinase (ALK). ROS protooncogene 1-encoded kinase (ROS1) of the tyrosine kinase insulin receptor class and MET proto-oncogene-encoded kinase of the hepatocyte growth factor receptor (HGFR) class are other kinases targeted by crizotinib.When approved in 2011, crizotinib was the first drug specifically targeting NSCLC patients. However, resistance to crizotinib was usually observed in approximately 8 months after initial application and more than half of crizotinib-treated patients experienced gastrointestinal side effects. In 2016,crizotinib was additionally approved for ROS1-positive NSCLC by FDA.
Crizotinib (Xalkori) is an oral receptor tyrosine kinase inhibitor indicated for the treatment of patients with advanced or metastatic non-small cell lung cancer (NSCLC). Common side effects with Xalkori use include upper respiratory infection, nausea, vomiting, stomach pain, decreased appetite, insomnia, dizziness, tired feeling, diarrhea, constipation, rash or itching, cold symptoms (stuffy nose, sneezing, sore throat), numbness or tingling, or swelling in your hands or feet.