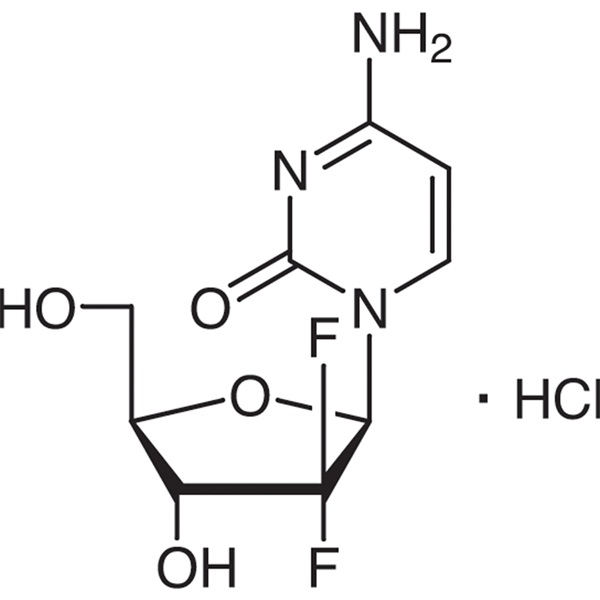
Gemcitabine Hydrochloride CAS 122111-03-9 API USP35 Standard
Ruifu Chemical is the leading manufacturer of Gemcitabine Hydrochloride (CAS: 122111-03-9) with high quality, commercial production. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Gemcitabine Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Gemcitabine Hydrochloride |
| Synonyms | Gemcitabine HCl; 2’-Deoxy-2’,2’-Difluorocytidine Hydrochloride; dFdC; dFdCyd; Gemzar; LY188011 Hydrochloride; Gemcitera; Gemsar |
| CAS Number | 122111-03-9 |
| Related CAS | 95058-81-4 - Free Base |
| Stock Status | In Stock, Production Capacity 5 Tons |
| Molecular Formula | C9H12ClF2N3O4 |
| Molecular Weight | 299.66 |
| Melting Point | >250℃ |
| Shipping Condition | Under Ambient Temperature |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | White Crystalline Powder, Odorless | Complies |
| Solubility | Soluble in Water, slightly soluble in methanol, practically insoluble in acetone |
Complies |
| Identifiaction IR | IR spectrum should be concordant to that of the reference standard |
Complies |
| Identifiaction Chloride | Positive. It meets the requirements of the tests for chloride | Complies |
| Appearance of Solution | Solution S in clear and not more intensely colored than reference solution BY7 |
Complies |
| pH | 2.0~3.0 | 2.6 |
| Specific Rotation [α]20/D | +43.0° to +50.0° | +47.5° |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Loss on Drying | ≤1.00% | 0.3% |
| Residue on Ignition | ≤0.10% | 0.03% |
| Related Substances | ||
| Cytosine | ≤0.10% | 0.01% |
| α-Isomer | ≤0.10% | 0.01% |
| Any Other Impurity | ≤0.10% | 0.04% |
| Total Impurities | ≤0.20% | 0.1% |
| Residual Solvents | ||
| Methanol | ≤0.30% | Not Detected |
| Toluene | ≤0.01% | Not Detected |
| Dichloromethane | ≤0.01% | Not Detected |
| Acetone | ≤0.50% | 0.1% |
| Assay | 97.5%~101.5% (Calculated on Dried Base) | 99.9% |
| Conclusion | Conforms to USP35 Standard | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Avoid exposure to direct sunlight, moisture and excessive heat.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Risk Codes R21 - Harmful in contact with skin
R36/38 - Irritating to eyes and skin.
R46 - May cause heritable genetic damage
R62 - Possible risk of impaired fertility
R63 - Possible risk of harm to the unborn child
Safety Description S25 - Avoid contact with eyes.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37 - Wear suitable protective clothing and gloves.
S53 - Avoid exposure - obtain special instructions before use.
WGK Germany 3
RTECS HA3840000
HS Code 2942000000
Gemcitabine Hydrochloride (CAS: 122111-03-9) is a synthetic novel difluoro nucleoside drug that is anti-metabolic and antineoplastic. It is researched and developed by the Eli Lilly and Company and approved to be listed in South Africa, Sweden, the Netherlands, Australia and other countries in 1995. The United States Food and Drug Administration (FDA) approved it as the first-line therapy for the clinical treatment of non-small cell lung cancer and pancreatic cancer.
In recent years, new drugs such as Gemcitabine, Paclitaxel, Docetaxel, Vinorebine are effective drugs for the treatment of non-small cell lung cancer (abbreviated NSCLC). Compared with traditional chemotherapy drugs, these drugs have the advantages of high curative effect and low toxicity. Gemcitabine Hydrochloride is a new generation of anti-metabolites drug and a type of special medicine for cell cycle, playing a major role in DNA synthesis phase, namely S phase of cells. Under certain conditions, this medicine can prevent progression of cells from G1 phase to S phase, and have a strong anti-cancer activity non-small cell lung cancer(NSCLC). Foreign studies have shown that the efficient of the single treatment for NSCLC with Gemcitabine Hydrochloride only is about 18%~35%, while combined the treatment with cisplatin the efficiency for NSCLC is 41.7%. In advanced NSCLC, effective rate of carboplatin is 16%, which is similar to cisplatin, but has low toxicity, especially for gastrointestinal reactions, bone marrow suppression and the toxic reaction of kidney and nerve ending. In combination with carboplatin, both of them have mutual coordination and additive effect, and can produce higher curative effects.
Gemcitabine Hydrochloride
C9H11F2N3O4·HCl 299.66
Cytidine, 2′-deoxy-2′,2′-difluro-, monohydrochloride.
2′-Deoxy-2′,2′-difluorocytidine monohydrochloride ( β-isomer) [122111-03-9].
» Gemcitabine Hydrochloride contains not less than 97.5 percent and not more than 101.5 percent of C9H11F2N3O4·HCl, calculated on the as-is basis.
[Caution-Gemcitabine Hydrochloride is a potent cytotoxic agent. Great care should be taken to prevent inhaling particles and exposing the skin to it.]
Packaging and storage-Preserve in tight containers.
Labeling-Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference standards <11>-
USP Cytosine RS
USP Endotoxin RS
USP Gemcitabine Hydrochloride RS
Identification-
A: Infrared Absorption <197K>.
B: It meets the requirements of the tests for Chloride <191>.
Specific rotation <781S>: between +43 and +50, at 20.
Test solution: 10 mg per mL.
pH <791>: between 2.0 and 3.0, in a solution containing 10 mg per mL.
Residue on ignition <281>: not more than 0.1%.
Heavy metals, Method I <231>: 0.001%.
Chromatographic purity-
Solution A- Proceed as directed for Mobile phase in the Assay.
Solution B-Prepare filtered and degassed methanol.
Mobile phase-Use variable mixtures of Solution A and Solution B as directed under Chromatographic system. Make adjustments, if necessary (see System Suitability under Chromatography 621).
System suitability solution-Proceed as directed in the Assay.
Standard solution-Dissolve an accurately weighed quantity of USP Gemcitabine Hydrochloride RS and USP Cytosine RS in water, and dilute quantitatively, and stepwise if necessary, to obtain a solution having a known concentration of about 2 µg per mL of each.
Test solution-Transfer about 50 mg of Gemcitabine Hydrochloride, accurately weighed, to a 25-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Chromatographic system (see Chromatography 621)-Proceed as directed under Assay. The chromatograph is programmed as follows.
Time (minutes) Solution A (%) Solution B (%) Elution
0–8 97 3 isocratic
8–13 97®50 3®50 linear gradient
13–20 50 50 isocratic
20–25 50®97 50®3 re-equilibration
Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.5 for gemcitabine -anomer and 1.0 for gemcitabine; the resolution, R, between gemcitabine -anomer and gemcitabine is not less than 8.0; and the tailing factor for gemcitabine is not more than 1.5. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.1 for cytosine and 1.0 for gemcitabine; the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Separately inject a volume (about 20 µL) of the Standard solution and Test solution into the chromatograph, record the chromatogram, and measure all of the peak responses. Calculate the percentage of cytosine in the portion of Gemcitabine taken by the formula:
2.5(Cc / W)(rt / rs)
in which Cc is the concentration of USP Cytosine RS in the Standard solution, in µg per mL; W is the weight, in mg, of Gemcitabine taken; rt is the peak response for cytosine in the Test solution; and rs is the response for cytosine in the Standard solution: not more than 0.1% of cytosine is found. Calculate the percentage of each impurity other than cytosine in the portion of Gemcitabine taken by the formula:
2.5(Cs / W)(ri / rs)
in which Cs is the concentration of USP Gemcitabine Hydrochloride RS in the Standard solution, in µg per mL; W is the weight, in mg, of Gemcitabine taken; ri is the peak response for each impurity in the Test solution; and rs is the response due to gemcitabine in the Standard solution: not more than 0.1% of gemcitabine -anomer or any other individual impurity is found; and the sum of all impurities is not more than 0.2%. Exclude from the sum of all impurities any peaks that are below the limit of quantitation (0.02%).
Other requirements-Where the label states that Gemcitabine Hydrochloride is sterile, it meets the requirements for Bacterial endotoxins and Sterility under Gemcitabine for Injection. Where the label states that Gemcitabine Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Gemcitabine for Injection.
Assay-
Mobile phase-Prepare a filtered and degassed solution containing 13.8 g of monobasic sodium phosphate and 2.5 mL of phosphoric acid in 1000 mL of water. [note-The pH of this solution is between 2.4 and 2.6. ]
System suitability solution-Transfer about 10 mg of Gemcitabine Hydrochloride to a small vial, add 4 mL of a solution containing 168 mg of potassium hydroxide per mL of methanol, cap tightly, and sonicate. Heat at 55 for 6 to 16 hours, allow to cool, and transfer the contents to a 100-mL volumetric flask with successive washes of 1% (v/v) phosphoric acid. Dilute with 1% phosphoric acid to volume, and mix. [note-This solution contains about 0.02 mg per mL of gemcitabine α-anomer.]
Standard preparation-Dissolve an accurately weighed quantity of USP Gemcitabine Hydrochloride RS in water, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having a known concentration of about 0.1 mg per mL.
Assay preparation-Transfer about 20 mg of Gemcitabine Hydrochloride, accurately weighed, to a 200-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 275-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.2 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between the gemcitabine -anomer and gemcitabine is not less than 8.0; and the tailing factor determined from gemcitabine is not more than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 1.0%.
Procedure-Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C9H11F2N3O4·HCl in the portion of Gemcitabine Hydrochloride taken by the formula:
200C(rU / rS)
in which C is the concentration, in mg per mL, of USP Gemcitabine Hydrochloride RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
-
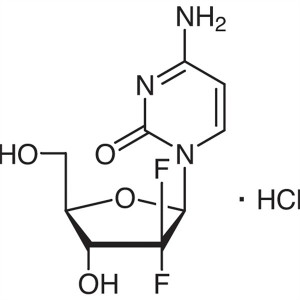
Gemcitabine Hydrochloride CAS 122111-03-9 API U...
-
Gemcitabine CAS 95058-81-4 Assay 98.0~102.0%
-
Cefotiam Hydrochloride CAS 66309-69-1 API USP S...
-
Doxorubicin Hydrochloride CAS 25316-40-9 API US...
-
Enalapril Maleate CAS 76095-16-4 Assay 98.0~102...
-
Guanfacine Hydrochloride Guanfacine HCl CAS 291...
-
Irinotecan Hydrochloride CAS 100286-90-6 Purity...
-
Levetiracetam LEV CAS 102767-28-2 Assay 98.0%~1...
-
Naltrexone Hydrochloride CAS 16676-29-2 API USP...
-
Noscapine Hydrochloride Hydrate CAS 912-60-7 AP...
-
Levodopa (L-DOPA) CAS 59-92-7 99.0~100.5% USP B...
-
Darunavir CAS 206361-99-1 Anti-HIV Purity ≥99.0...
-
Ezetimibe CAS 163222-33-1 Purity 98.5%~102.0% (...
-
Lasofoxifene Tartrate CAS 190791-29-8 Chiral Pu...
-
Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...