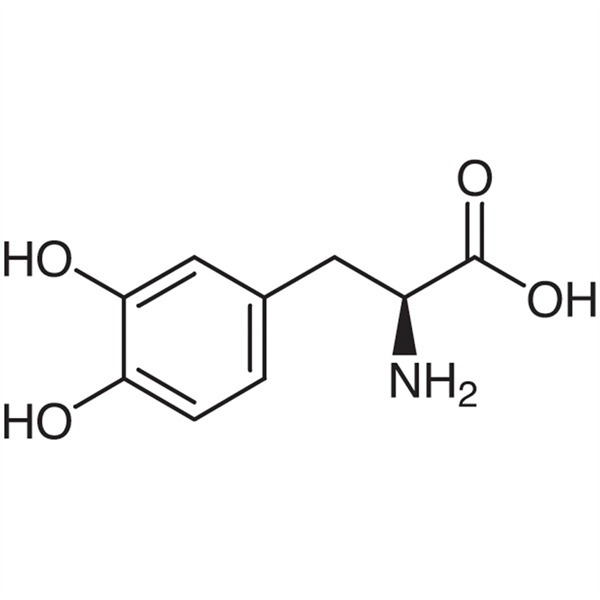
Levodopa (L-DOPA) CAS 59-92-7 99.0~100.5% USP BP EP Standard Anti-Parkinson’s Disease High Purity
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Levodopa (L-DOPA) (CAS: 59-92-7) with high quality, Anti-Parkinson's Disease. As one of the largest Levodopa suppliers in China, Ruifu Chemical supplys qualified Levodopa up to international standards, such as AJI, USP, EP, BP and IP standard. Production capacity 300 tons per year. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in Levodopa, Please contact: alvin@ruifuchem.com
| Chemical Name | Levodopa |
| Synonyms | L-DOPA; 3-(3,4-Dihydroxyphenyl)-L-Alanine; L-3-(3,4-Dihydroxyphenyl)alanine; 3,4-L-Dihydroxyphenylalanine; 3-Hydroxy-L-Tyrosine; L-3-Hydroxytyrosine; H-Tyr(3-OH)-OH |
| CAS Number | 59-92-7 |
| CAT Number | RF-API55 |
| Stock Status | In Stock, Production Capacity 300 Tons per Year |
| Molecular Formula | C9H11NO4 |
| Molecular Weight | 197.19 |
| Melting Point | 276.0~278.0℃(lit.) |
| Sensitive | Light Sensitive, Air Sensitive |
| Solubility in Water | Slightly Soluble in Water |
| Solubility | Insoluble in Chloroform, Ethanol, Benzene, Ether. |
| Solubility in 1mol/L HCl | Dissolves in Dilute Hydrochloric Acid. Almost Transparency |
| Stability | Stable. Incompatible with Strong Oxidizing Agents. Light and Air Sensitive |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Milk White Crystalline Powder |
| Identification | Infrared Absorption Spectrum |
| Optical Rotation [α]20/D | -1.27° to -1.34° |
| Appearance of Solution | Passes Test |
| Particle Size | 100% Pass Through 80 |
| Related Substances | |
| L-Tyrosina | ≤0.10% |
| Levodopareted Compound | ≤0.10% |
| 3-Methoxytyrosine | ≤0.50% |
| Total Impurities | ≤1.00% |
| Unknow Impurities | ≤0.10% |
| UV Spectrum MaxE1%1cm | 137~147 (280nm) |
| Heavy Metals (as Pb) | ≤10ppm |
| Loss on Drying | ≤1.00% (at 105℃ for 4 Hours) |
| Residue on Ignition (Sulfated) | ≤0.10% |
| Assay | 99.0%~100.5% |
| PH | 4.5~7.0 (0.10g in 10ml of H2O Shaking for 15 Minutes) |
| Test Standard | AJI/USP/BP/EP/IP/JP Standard |
| Usage | Active Pharmaceutical Ingredient (API); Anti-Parkinson's Disease |
Optical Rotation [EP]
Dissolve a quantity equivalent to 0.200g of the dreied substance and 5g of hexamethylenetetramine R in 10ml of 1mol/L hydrochloric acid and dilute to 25.oml with the same acid. Allow the solution to stand protected from light for 3h. The angle of optical rotation is -1.27° to -1.34°
Appearance of Solution
Disslove 1.0g in 1mol/L hydrochloric acid and dilute to 25ml with the same solvnet. The solution is not more intensely coloured than reference solution BY6.
Related Substances [EP]
Examine by thin-layer chromatography, using cellulose for chromatography R as the coating substance.
Test solution-Dissolve 0.1g of the substance to be examined in 5ml of anhydrous formic acid R and dilute to 10ml with methanol R. Prepare immediately before use.
Reference solution (a)-Dilute 0.5ml of the test solution to 100ml with methanol R.
Reference solution (b)-Dissolve 30mg of tyrosine R in 1ml of anhydrous formic acid R and dilute to 100ml with methanol R. Mix 1ml of this solution with 1ml of the test solution.
Apply separately to the plate as bands 20mm long, 1oμl of the test solution, 10μl of reference solution (a) and 20μl of reference solution (b). Dry in a current of air. Develop over a path od 15cm using a mixture of 50 volumes of butanol R, 25 volumes of glacial acetic acid R and 25 volumes of water. Dry the plate in a current of warm air and spray with a freshly prepared mixture of equal volumed of a 10 percent m/v solution of ferric chloride R and a 5 percent m/v solution of postassium ferricyanide R. Examine the chromatoframs immediately. Any spot in the chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot in the chromatogram ibtained with reference solution (a) shows. The test is not valid unless the chromatogram obtained with reference solution (b) shows, above the principal spot, a distinct spot which is more intense than the spot in the chromatogram obtained with reference solution (a).
UV Spectrum [EP]
Disslove 30.0mg in 0.1mol/L hydrochloric acid and dilute to 100.oml with the same acid. Dilute 10.0ml of this solution to 100.0ml with 0.1mol/L hydrochloric acid. Examined between 230nm and 350nm, the solution shows a single absorption maximum at 280nm. The specific absorbance at this maximum is 137 to 147, calculated with reference to the dried substance.
Loss on Drying
Take this product, dry to constant weight at 105°C, weight loss shall not exceed 1.0% (General rule 0831).
Residue on Ignition (Sulfated)
Take l.0g of Levodopa and check it according to law (General rule 0841). The residue left shall not exceed 0.1%.
Heavy Metals
The residue left under the item of taking the ignition residue shall not contain more than 10 parts per million of heavy metal when examined by law (General Principles 0821, Law II).
pH Test
0.10g in 10ml of H2O shaking for 15 minutes.
Content determination
Take this product about 0.lg, precision weighing, add anhydrous formic acid 2ml to dissolve, add glacial acetic acid 20ml, shake, add crystal violet indicator 2 drops, with perchloric acid titration solution (0.1 mol/L) titration to the solution is green, and the result of the titration is corrected with a blank test. Each 1 ml of perchloric acid titration solution (0.1 mol/L) corresponds to 19.72mg of C9H11N04.
JP17 Test Method
Levodopa, when dried, contains not less than 98.5% of Levodopa (C9H11NO4).
Description Levodopa occurs as white or slightly grayish white, crystals or crystalline powder. It is odorless. It is freely soluble in formic acid, slightly soluble in water, and practically insoluble in ethanol (95). It dissolves in dilute hydrochloric acid. The pH of a saturated solution of Levodopa is between 5.0 and 6.5. Melting point: about 275℃ (with decomposition).
Identification
(1) To 5 mL of a solution of Levodopa (1 in 1000) add 1mL of ninhydrin TS, and heat for 3 minutesin a water bath: a purple color develops.
(2) To 2 mL of a solution of Levodopa (1 in 5000) add 10 mL of 4-aminoantipyrine TS, and shake: a red color develops.
(3) Dissolve 3 mg of Levodopa in 0.001 mol/L hydrochloric acid TS to make 100 mL. Determine the absorption spectrum of the solution as directed under Ultravioletvisible Spectrophotometry <2.24>, and compare the spectrum withthe Reference Spectrum: both spectra exhibit similar intensities of absorption at the same wavelengths.
Absorbance <2.24> E 1%1cm (280 nm): 136-146 (after drying, 30 mg, 0.001 mol/L hydrochloric acid TS, 1000 mL).
Optical Rotation <2.49> [a]20D:-11.5° ~-13.0°(after drying, 2.5 g, 1 mol/L hydrochloric acid TS, 50 mL, 100 mm).
Purity
(1) Clarity and color of solution-Dissolve 1.0 g of Levodopa in 20 mL of 1 mol/L hydrochloric acid TS: the solution is clear and colorless.
(2) Chloride <1.03>-Dissolve 0.5 g of Levodopa in 6 mLof dilute nitric acid, and add water to make 50 mL. Perform the test using this solution as the test solution. Prepare the control solution with 0.30 mL of 0.01 mol/L hydrochloricacid VS (not more than 0.021%).
(3) Sulfate <1.14>-Dissolve 0.40 g of Levodopa in 1 mL of dilute hydrochloric acid and 30 mL of water, and add water to make 50 mL. Perform the test using this solution as the test solution. Prepare the control solution with 0.25 mL of 0.005 mol/L sulfuric acid VS (not more than 0.030%).
(4) Heavy Metals <1.07>-Proceed with 1.0 g of Levodopa according to Method 2, and perform the test. Preparethe control solution with 2.0 mL of Standard Lead Solution(not more than 20 ppm).
(5) Arsenic <1.11>-Dissolve 1.0 g of Levodopa in 5 mLof dilute hydrochloric acid, and perform the test with this solution as the test solution (not more than 2 ppm).
(6) Related substances-Dissolve 0.10 g of Levodopa in10 mL of sodium disulfite TS, and use this solution as the sample solution. Pipet 1 mL of the sample solution, add sodium disulfite TS to make exactly 25 mL. Pipet 1 mL of this solution, add sodium disulfite TS to make exactly 20 mL,and use this solution as the standard solution. Perform thetest with these solutions as directed under Thin-layer Chro-matography<2.03>. Spot 5mL each of the sample solutionand standard solution on a plate of cellulose for thin-layerchromatography. Develop the plate with a mixture of 1-butanol, water, acetic acid (100) and methanol (10:5:5:1) toa distance of about 10 cm, and air-dry the plate. Sprayevenly a solution of ninhydrin in acetone (1 in 50) on the plate and heat at 90℃ for 10 minutes: the spots other thanthe principal spot from the sample solution are not more intense than the spot from the standard solution.
Loss on Drying <2.41> Not more than 0.30%(1 g, 105℃,3 hours).
Residue on Ignition <2.44> Not more than 0.1% (1 g).
Assay Weigh accurately about 0.3 g of Levodopa, previously dried, dissolve in 3 mL of formic acid, add 80 mL of acetic acid (100), and titrate <2.50> with 0.1 mol/L perchloric acid VS until the color of the solution changes from purple through blue-green to green (indicator: 3 drops of crystalviolet TS). Perform a blank determination, and make anynecessary correction.
Each mL of 0.1 mol/L perchloric acid VS=19.72 mg of C9H11NO4
Containers and storage Containers-Tight containers.
Storage-Light-resistant.
| Hazard Codes | Xn | RTECS | AY5600000 |
| Risk Statements | 22-36/37/38-20/21/22 | F | 10-23 |
| Safety Statements | 26-36-24/25 | TSCA | Yes |
| WGK Germany | 3 | HS Code | 2922509099 |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Light and air sensitive. Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light, air and moisture.


Levodopa (L-DOPA) (CAS 59-92-7) is used in the treatment of Parkinson's Disease. Levodopa (L-DOPA), the most reliable and effective drug used in the treatment of parkinsonism, can be considered a form of replacement therapy. Levodopa is widely used for treatment of all types of parkinsonism except those associated with antipsychotic drug therapy. Levodopa is the biochemical precursor of dopamine. It is used to elevate dopamine levels in the neostriatum of parkinsonian patients. Dopamine itself does not cross the blood-brain barrier and therefore has no CNS effects. However, Levodopa , as an amino acid, is transported into the brain by amino acid transport systems, where it is converted to dopamine by the enzyme L-aromatic amino acid decarboxylase. If Levodopa is administered alone, it is extensively metabolized by L-aromatic amino acid decarboxylase in the liver, kidney, and gastrointestinal tract. To prevent this peripheral metabolism, Levodopa is coadministered with Carbidopa (Sinemet), a peripheral decarboxylase inhibitor. The combination of Levodopa with Carbidopa lowers the necessary dose of Levodopa and reduces peripheral side effects associated with its administration. Levodopa is widely used for treatment of all types of parkinsonism except those associated with antipsychotic drug therapy. However, as parkinsonism progresses, the duration of benefit from each dose of Levodopa may shorten (wearing-off effect). Patients can also develop sudden, unpredictable fluctuations between mobility and immobility (on-off effect). In a matter of minutes, a patient enjoying normal or nearly normal mobility may suddenly develop a severe degree of parkinsonism. These symptoms are likely due to the progression of the disease and the loss of striatal dopamine nerve terminals.
-
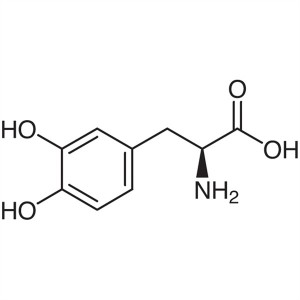
Levodopa (L-DOPA) CAS 59-92-7 99.0~100.5% USP B...
-
Cefotiam Hydrochloride CAS 66309-69-1 API USP S...
-
Doxorubicin Hydrochloride CAS 25316-40-9 API US...
-
Enalapril Maleate CAS 76095-16-4 Assay 98.0~102...
-
Guanfacine Hydrochloride Guanfacine HCl CAS 291...
-
Naltrexone Hydrochloride CAS 16676-29-2 API USP...
-
Levetiracetam LEV CAS 102767-28-2 Assay 98.0%~1...
-
Palonosetron Hydrochloride CAS 135729-62-3 Puri...
-
Noscapine Hydrochloride Hydrate CAS 912-60-7 AP...
-
Irinotecan Hydrochloride CAS 100286-90-6 Purity...
-
Ropivacaine Hydrochloride Monohydrate CAS 13211...
-
Tetracaine Hydrochloride CAS 136-47-0 API USP S...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...
-
Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-
Esmolol Hydrochloride CAS 81161-17-3 Purity ≥99...