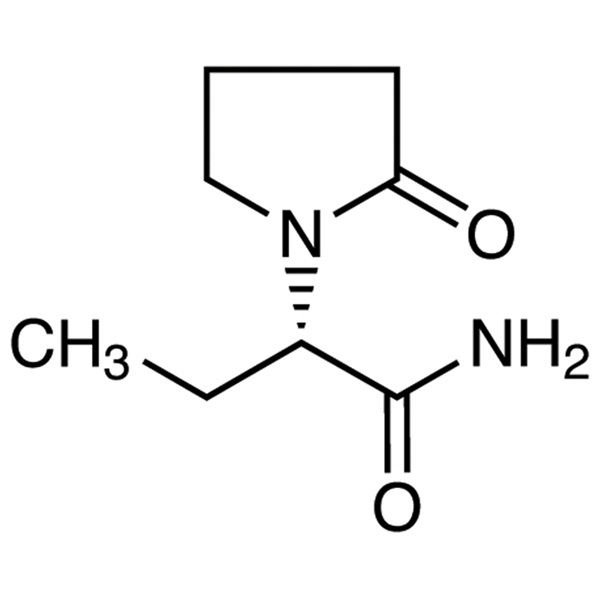
Levetiracetam LEV CAS 102767-28-2 Assay 98.0%~102.0% USP EP Standard
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Levetiracetam (CAS: 102767-28-2) with high quality and stable quality, API, USP/EP standard, third-generation antiepileptic drug. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Levetiracetam, Please contact: alvin@ruifuchem.com
| Chemical Name | Levetiracetam |
| Synonyms | LEV; (S)-2-(2-Oxo-1-pyrrolidinyl)butyramide; UCB-L059; (S)-alpha-Ethyl-2-Oxo-1-Pyrrolidineacetamide; (2S)-2-(2-Oxopyrrolidin-1-yl)butanamide |
| CAS Number | 102767-28-2 |
| Stock Status | In Stock, Production Capacity 500 Tons per Year |
| Molecular Formula | C8H14N2O2 |
| Molecular Weight | 170.21 |
| Melting Point | 116.0~119.0℃ |
| Density | 1.168g/cm3 |
| Solubility | Soluble in Acetone |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | Antiepileptic; API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystals Powder |
| Identification | IR |
| Appearance of Solution | Clear and not more intensely coloured than BY6 |
| Limit of Levetiracetam R-Enantiomer | ≤0.80% |
| Water (by K.F) | ≤0.50% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤0.001% |
| Related Substances | |
| Pyridin-2-ol | ≤0.025% |
| Levetiracetam Acid | ≤0.30% |
| Levetiracetam Related Compound A | ≤0.05% |
| Levetiracetam Related Compound B | ≤0.10% |
| Any Individual Unspecified Impurity | ≤0.05% |
| Total Unspecified Impurities | ≤0.10% |
| Total Impurities | ≤0.40% |
| Residual Solvents | Meet the Specification |
| Assay / Analysis Method | 98.0%~102.0% (Calculated on the anhydrous & solvent-free basis) |
| Test Standard | USP Standard; EP Standard |
| Application | API; Third-Generation Antiepileptic Drug |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry, well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Levetiracetam
C8H14N2O2 170.21
1-Pyrrolidineacetamide, -ethyl-2-oxo-, (S)-;
()-(S)--Ethyl-2-oxo-1-pyrrolidineacetamide [102767-28-2].
DEFINITION
Levetiracetam contains NLT 98.0% and NMT 102.0% of C8H14N2O2, calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• A. Infrared Absorption <197K>
• B. The retention time of the major peak for levetiracetam from the Sample solution corresponds to that of the levetiracetam S-enantiomer from the System suitability solution, as obtained in the test for Limit of Levetiracetam R-Enantiomer.
ASSAY
• Procedure
Buffer: 2.7 g/L of monobasic potassium phosphate in water. Adjust with 2% aqueous potassium hydroxide (w/v) to a pH of 5.5.
Solution A: Acetonitrile and Buffer (1:19)
Solution B: Acetonitrile
Mobile phase: See the gradient table below.
Time (min) Solution A (%) Solution B (%)
0 100 0
3 100 0
20 71 29
System suitability solution: 0.2 mg/mL of USP Levetiracetam RS and 0.08 mg/mL of USP Levetiracetam Related Compound A RS in Solution A. Prepare by first dissolving the required amount of USP Levetiracetam RS in a suitable volumetric flask. Add 10% of the flask volume of 0.1 N potassium hydroxide. Let the mixture react at room temperature for about 15 min, and then neutralize by adding 0.1 N hydrochloric acid at 10% of the flask volume. Add the required amount of USP Levetiracetam Related Compound A RS, sonicate to dissolve, dilute with Solution A to volume, and mix.
Standard solution: 0.1 mg/mL of USP Levetiracetam RS in Solution A
Sample solution: 0.1 mg/mL of Levetiracetam in Solution A
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 205 nm
Column: 4.6-mm × 15-cm; packing L1
Flow rate: 0.9 mL/min
Injection size: 10 µL
System suitability
Sample: System suitability solution
[Note-The relative retention times are given in Impurity Table 1.]
Suitability requirements
Relative standard deviation: NMT 1.0%
[Note-If system suitability criteria cannot be met, it is recommended that the column temperature be maintained at 20 to stabilize the system.]
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C8H14N2O2 in the portion of Levetiracetam taken:
Result = [(rU/rS) × (CS/CU) × 100] F
rU = peak response of levetiracetam from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
F = percentage of levetiracetam R-enantiomer from the test for Limit of Levetiracetam R-Enantiomer
Acceptance criteria: 98.0%-102.0% on the anhydrous and solvent-free basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.1%
• Heavy Metals, Method II <231>: 20 ppm
Organic Impurities
• Procedure 1: Limit of Levetiracetam Related Compound B
[Note-Perform this test only if levetiracetam related compound B is a known process impurity.]
Buffer: 1.22 g of sodium 1-decanesulfonate in 1 L of water containing about 1.3 mL of phosphoric acid. Adjust with 20% (w/v) potassium hydroxide to a pH of 3.0.
Mobile phase: Acetonitrile and Buffer (3:17)
System suitability solution: 2 mg/mL of USP Levetiracetam Related Compound B RS in Mobile phase
Standard solution: 0.002 mg/mL of USP Levetiracetam Related Compound B RS in Mobile phase
Sample solution: 2.0 mg/mL of Levetiracetam in Mobile phase
Chromatographic system
(See Chromatography 621, System Suitability.)
Mode: LC
Detector: UV 200 nm
Column: 4.6-mm × 25-cm; packing L1
Flow rate: 1.0 mL/min
Injection size
System suitability: 10 µL
Analysis: 50 µL
System suitability
Sample: System suitability solution
[Note-The retention time for levetiracetam related compound B is 9 min.]
Suitability requirements
Tailing factor: NMT 3.0
[Note-If a significant tailing of the levetiracetam related compound B peak is observed (greater than 3.0), it is recommended that the column temperature be maintained at 27 to stabilize the system.]
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levetiracetam related compound B in the portion of Levetiracetam taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of levetiracetam related compound B from the Sample solution
rS = peak response of levetiracetam related compound B from the Standard solution
CS = concentration of USP Levetiracetam Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
Mr1 = molecular weight of levetiracetam related compound B free base, 102.1
Mr2 = molecular weight of levetiracetam related compound B, 138.6
Acceptance criteria: NMT 0.10%
[Note-The amount of levetiracetam related compound B measured is to be included in the total impurities in the test for Organic Impurities, Procedure 2.]
• Procedure 2
Buffer, Solution A, Solution B, Mobile phase, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 0.005 mg/mL of USP Levetiracetam RS in Solution A
Sample solution: 5 mg/mL of Levetiracetam in Solution A
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Levetiracetam taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
F = relative response factor (see Impurity Table 1)
[Note-Disregard any peak with a relative retention time of 0.19 or less.]
Acceptance criteria
Individual impurities: See Impurity Table 1.
Total impurities: NMT 0.4%
Impurity Table 1
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Pyridin-2-ol a 0.37 1.0 0.025
Levetiracetam acidb 0.62 1.2 0.3
Levetiracetam 1.00 - -
Levetiracetam related compound Ac 1.25 0.35 0.05
Any individual unspecified impurity - 1.0 0.05
a Not included in the Total impurities limit.
b (S)-2-(2-Oxopyrrolidin-1-yl)butanoic acid. Included in the Total impurities limit.
c (S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide. Included in the Total impurities limit only if levetiracetam related compound B is a known process impurity.
SPECIFIC TESTS
• Water Determination, Method Ia <921>: NMT 0.5%
• Limit of Levetiracetam R-Enantiomer
Mobile phase: n-Hexane and dehydrated alcohol (4:1)
System suitability solution: 0.1 mg/mL of USP Levetiracetam Racemic Mixture RS in Mobile phase
Standard solution: 0.05 mg/mL of USP Levetiracetam RS in Mobile phase
Sample solution: 10 mg/mL of Levetiracetam in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 25-cm; 10-µm packing L51
Flow rate: 1.0 mL/min
Injection size: 20 µL
System suitability
Sample: System suitability solution
[Note-The relative retention times for levetiracetam R-enantiomer and levetiracetam S-enantiomer are 0.55 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 4.0 between the R- and S-enantiomers
[Note-If a loss of resolution (less than 4.0) is observed, it is recommended that the column temperature be maintained at 25 to stabilize the system.]
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of levetiracetam R-enantiomer in the portion of Levetiracetam taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of levetiracetam R-enantiomer from the Sample solution
rS = peak response of levetiracetam from the Standard solution
CS = concentration of USP Levetiracetam RS in the Standard solution (mg/mL)
CU = concentration of Levetiracetam in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.8%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers, and store at room temperature.
• USP Reference Standards <11>
USP Levetiracetam RS
USP Levetiracetam Racemic Mixture RS
A 1:1 mixture of levetiracetam S-enatiomer-(2S)-2-(2-oxopyrrolidin-1-yl)butanamide and levetiracetam R-enantiomer (2R)-2-(2-oxopyrrolidin-1-yl)butanamide.
USP Levetiracetam Related Compound A RS
(S)-N-(1-Amino-1-oxobutan-2-yl)-4-chlorobutanamide.
C8H14ClNO3 207.65
USP Levetiracetam Related Compound B RS
(S)-2-Aminobutanamide hydrochloride.
C4H10N2O·HCl 138.6

Levetiracetam (CAS: 102767-28-2), a derivative of pilacetam, is a novel third-generation antiepileptic drug approved by the US FDA in 1999. It was initially used for the adjunctive treatment of partial seizures in adults. In 2005, Levetiracetam was approved in oral tablets and solutions for the adjunctive treatment of partial seizures in children aged 4 years and older. It is mainly used for the additive treatment of partial seizures in adults and children over 4 years old, and can also be used only for partial seizures and systemic seizures in adults. It also has certain curative effect on myoclonic epilepsy in teenagers, refractory epilepsy, absent epilepsy in children and persistent epilepsy.