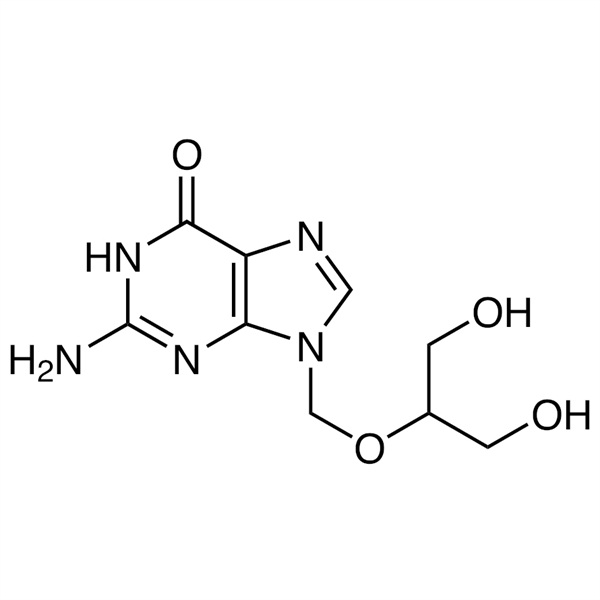
Ganciclovir CAS 82410-32-0 API BW 759 GCV Antiviral CMV Inhibitor High Quality
Manufacturer Supply with High Purity and Stable Quality
Chemical Name: Ganciclovir
CAS: 82410-32-0
Antiviral Agent for the Treatment of Cytomegalovirus (CMV) Infection
API High Quality, Commercial Production
| Chemical Name | Ganciclovir |
| Synonyms | GCV; BW 759; 9-[[2-Hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine; 2-Amino-9-(1,3-dihydroxypropan-2-yloxymethyl)-3H-purin-6-one; 2'-Nor-2'-Deoxyguanosine |
| CAS Number | 82410-32-0 |
| CAT Number | RF-API82 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C9H13N5O4 |
| Molecular Weight | 255.23 |
| Melting Point | 250℃ |
| Storage Temperature | 2-8℃ |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystalline Powder |
| Solubility | Very Slightly Soluble in Water |
| Identification | Ultraviolet Absorption, Infrared Absorption |
| Loss on Drying | ≤6.0% |
| Residue on Ignition | ≤0.10% |
| Related Substances | Ganciclovir Related Compound A ≤0.50% |
| Limit of Guanine | ≤0.50% |
| Residual Solvents (GC) | Ethanol ≤5000ppm |
| Heavy Metals | ≤10ppm |
| Absorption Coefficient | 516-548 at 252nm |
| Bacterial Endotoxins | ≤0.84EU/mg |
| Assay | 98.0%~102.0% (Titration) |
| Test Standard | Enterprise Standard; United States Pharmacopoeia (USP) Standard |
| Usage | API, CMV Inhibitor |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Ganciclovir (CAS 82410-32-0) belongs to the nucleoside antiviral drugs, being a kind of guanosine derivatives. Ganciclovir is a parenterally-active antiviral agent indicated for sight- or life-threatening cytomegalovirus (CMV) infections in immunocompromised patients. It has broad-spectrum, high-efficient inhibitory effects on herpes virus and is the first-choice drug for the treatment of cytomegalovirus infection with strong effect on hepatitis B virus and adenovirus as well. It is homologue of acyclovir (ACV) with its antiviral effect being similar as, but stronger than aciclovir, having especially strong inhibitory effects on cytomegalovirus associated with AIDS patients. It is clinical used for the treatment of induction phase and maintenance phase on immunocompromised patients (including AIDS patients) with concurrent cytomegalovirus retinitis. It can also be used for the prevention of cytomegalovirus disease for patients receiving organ transplantation or AIDS patients with positive results in cytomegalovirus serology test.
Ganciclovir (CAS 82410-32-0) is a highly efficient, low-toxicity and high-selectivity virus inhibitor developed by the Syntex Company (United States). It is the first drug that has been approved by the US FDA for the treatment of cytomegalovirus (CMV) infection. Syntex has been granted of the exclusive right of production. In June 1988, this tablet has been approved for the first time to be listed in the UK, followed by being successively approved by France, the United States, Japan, and West Germany, Italy and Canada and other countries. Until the end of June 1999, it has been approved in more than 70 countries and regions for the prevention of immunodeficiency patients and the cytomegalovirus infection of patients of organ transplantation. In 2002, Ganciclovir tablets have obtained approval of the FDA, becoming available now.
-
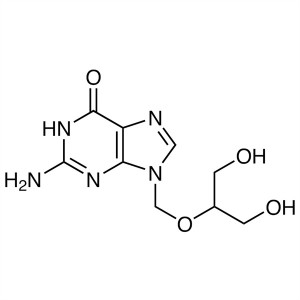
Ganciclovir CAS 82410-32-0 API BW 759 GCV Antiv...
-
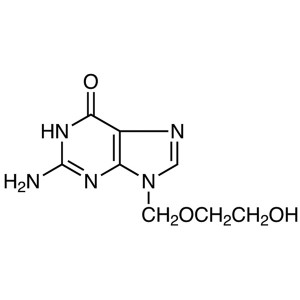
Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPL...
-
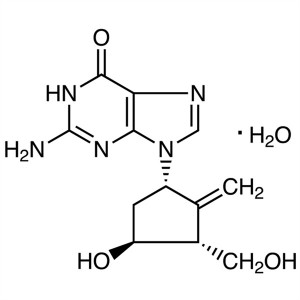
Entecavir Monohydrate CAS 209216-23-9 API Facto...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Acotiamide Hydrochloride Trihydrate CAS 773092-...
-
Baloxavir Marboxil CAS 1985606-14-1 API Factory...
-
Azithromycin Dihydrate CAS 117772-70-0 Assay 94...
-
Azelastine Hydrochloride CAS 79307-93-0 Assay 9...
-
Ibrutinib CAS 936563-96-1 Purity >99.5% (HPLC) API
-
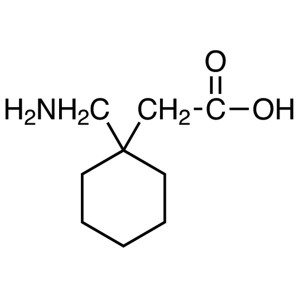
Gabapentin CAS 60142-96-3 Purity >99.5% (HPLC) ...
-
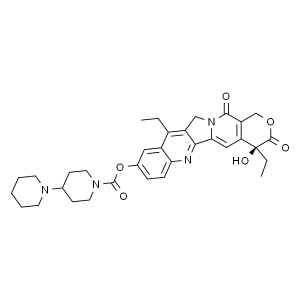
Irinotecan Free Base CAS 97682-44-5 Assay ≥99.0...
-
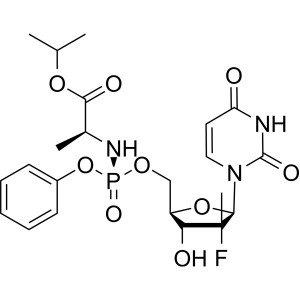
Sofosbuvir CAS 1190307-88-0 Purity ≥99.0% (HPLC)
-
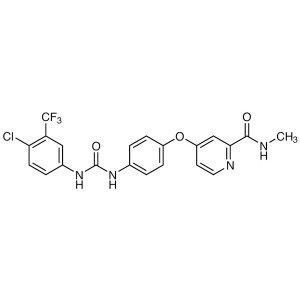
Sorafenib CAS 284461-73-0 Purity ≥99.0% (HPLC) ...