Acotiamide Hydrochloride Trihydrate CAS 773092-05-0 API Manufacturer High Purity
Manufacturer with High Purity and Stable Quality
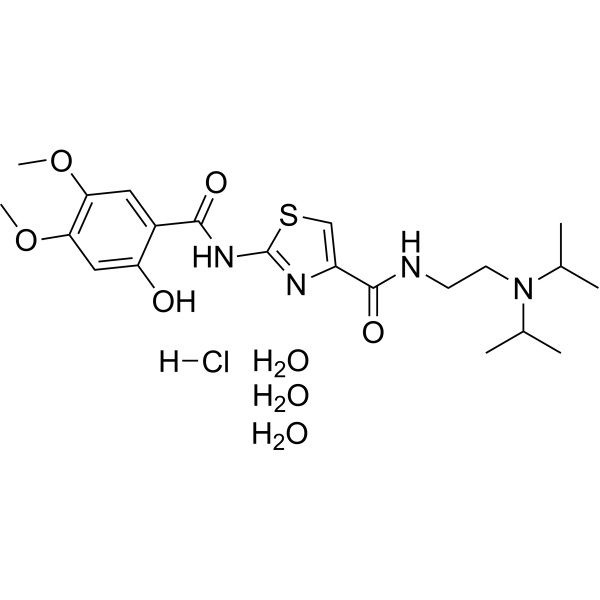
Chemical Name: Acotiamide Hydrochloride Trihydrate
CAS: 773092-05-0
In the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia.
API High Quality, Commercial Production
| Chemical Name | Acotiamide Hydrochloride Trihydrate |
| Synonyms | YM-443, Z-338 |
| CAS Number | 773092-05-0 |
| CAT Number | RF-API34 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C21H31ClN4O5S |
| Molecular Weight | 487.013 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Powder |
| Purity / Analysis Method | ≥98.0% (HPLC) |
| Residual Solvent Ethanol | ≤0.50% |
| Moisture (K.F) | 9.0%~12.0% |
| Individual Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Residue on Ignition | ≤0.20% |
| 1H NMR | Corresponds to the Structure |
| Test Standard | Enterprise Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Acotiamide Hydrochloride Trihydrate (CAS: 773092-05-0) with high quality. Acotiamide, also known as YM-443 and Z-338, is a drug approved in Japan for the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia. It acts as an acetylcholinesterase inhibitor. Note: The Approved drug API is a cotiamide HCl trihydrate (1:1:3). Acotiamide Hydrochloride is the hydrochloride salt form of acotiamide, a prokinetic agent with gastrointestinal (GI) motility-enhancing activity. It is a new orally active selective acetylcholinesterase inhibitor. Acotiamide monohydrochloride trihydrate enhances acetylcholine released by enteric neurons through muscarinic receptor antagonism and acetylcholinesterase (AChE) inhibition, thereby enhancing gastric emptying and gastric accommodation.
-
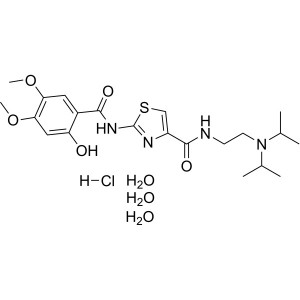
Acotiamide Hydrochloride Trihydrate CAS 773092-...
-
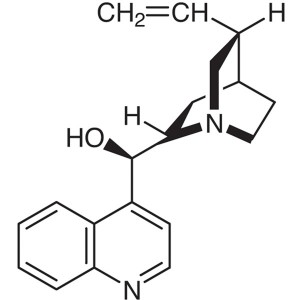
Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...
-
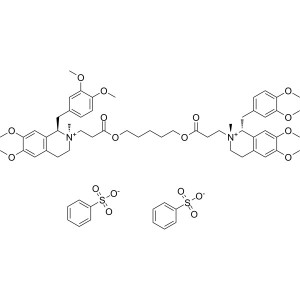
Cisatracurium Besylate CAS 96946-42-8 Assay 95....
-
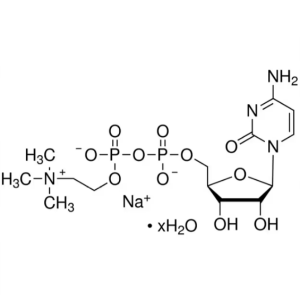
Citicoline Sodium Salt Hydrate CAS 33818-15-4 A...
-
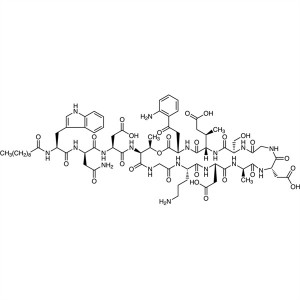
Daptomycin CAS 103060-53-3 Purity ≥95.0% API Fa...
-
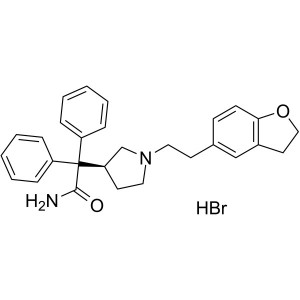
Darifenacin Hydrobromide CAS 133099-07-7 Assay ...
-
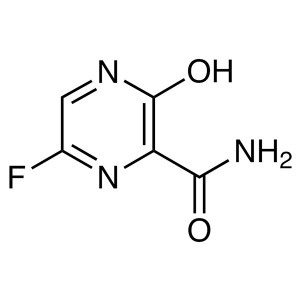
Favipiravir CAS 259793-96-9 T-705 Purity ≥99.0%...
-
Glimepiride CAS 93479-97-1 Assay 98.0%~102.0% A...
-
Ibrutinib CAS 936563-96-1 Purity >99.5% (HPLC) API
-
Imatinib Mesylate CAS 220127-57-1 Assay 98.0%~1...
-
Lapatinib Base CAS 231277-92-2 Purity ≥99.0% (H...
-
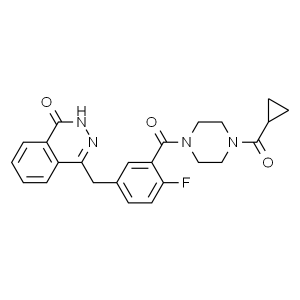
Olaparib AZD-2281 CAS 763113-22-0 Purity ≥99.0%...
-
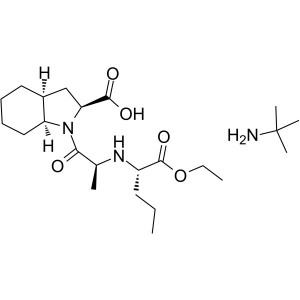
Perindopril Erbumine CAS 107133-36-8 Purity >99...
-
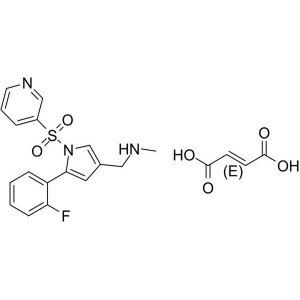
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
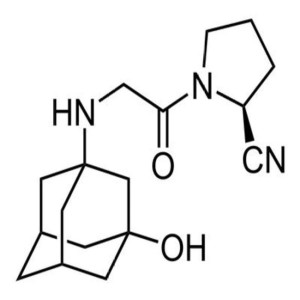
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...
-
Molnupiravir (EIDD-2801) CAS 2349386-89-4 COVID...