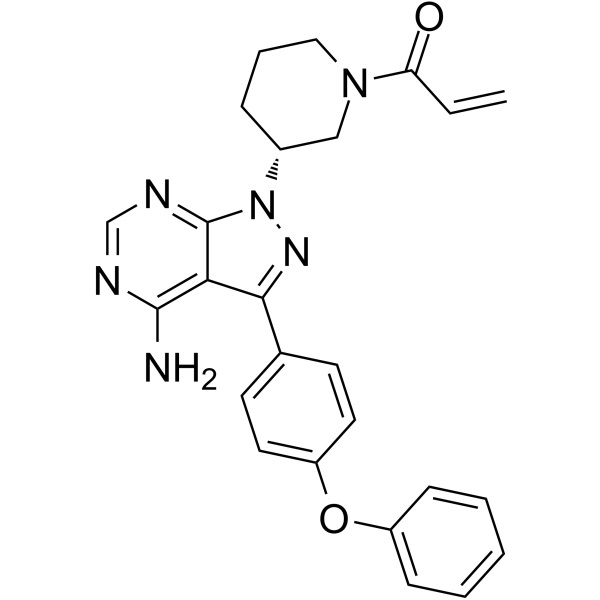
Ibrutinib CAS 936563-96-1 Purity >99.5% (HPLC) API
| Chemical Name | Ibrutinib |
| Synonyms | 1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one; PCI-32765 |
| CAS Number | 936563-96-1 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C25H24N6O2 |
| Molecular Weight | 440.50 |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Crystal Powder |
| Identification | IR; HPLC |
| Loss on Drying | <0.50% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals (as Pb) | ≤20ppm |
| Any Single Impurity | ≤0.50% |
| Total Impurities | <0.50% |
| Purity / Analysis Method | >99.5% (HPLC) |
| Test Standard | Enterprise Standard |
| Usage | API |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.

Ibrutinib (CAS: 936563-96-1) is an inhibitor of Bruton tyrosine kinase (BTK) for the treatment of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Both MCL and CLL belong to B-cell non-Hodgkin's lymphoma, which is refractory and prone to relapse. The commonly used chemoimmunotherapy is not targeted, and grade 3 or 4 adverse reactions often occur. Ibrutinib can combine with BTK, which is necessary for the formation, differentiation, communication and survival of B lymphocytes, and irreversibly inhibit the activity of BTK, effectively inhibit the proliferation and survival of tumor cells. In addition, it is rapidly absorbed after oral administration, the maximum plasma concentration is reached 1~2h, and the adverse reactions are grade 1 or 2, which will become a new option for the treatment of CLL and MCL. On November 13, 2013, the U.S. FDA to accelerate the approved Johnson & Johnson company and the United States Imbruvica (common name: Ibrutinib) for the treatment of mantle cell lymphoma (MCL). Ibrutinib, was granted breakthrough Therapy status by the FDA in February 2013 and was approved for MCL on November 13, 2013 and CLL on February 12, 2014, respectively.
-
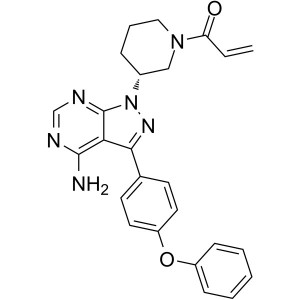
Ibrutinib CAS 936563-96-1 Purity >99.5% (HPLC) API
-
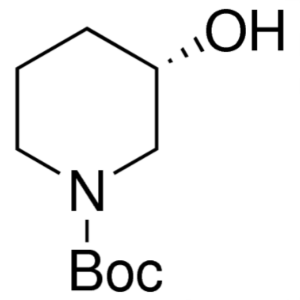
(S)-1-Boc-3-Hydroxypiperidine CAS 143900-44-1 I...
-
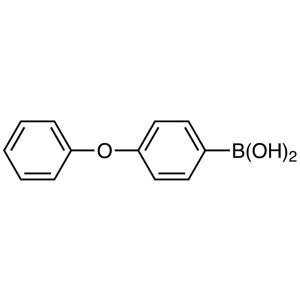
4-Phenoxyphenylboronic Acid CAS 51067-38-0 Ibru...
-
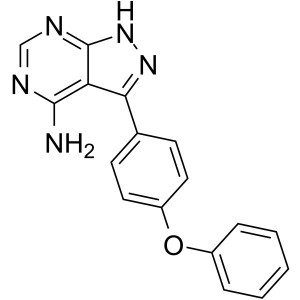
Ibrutinib Deacryloylpiperidine CAS 330786-24-8 ...
-
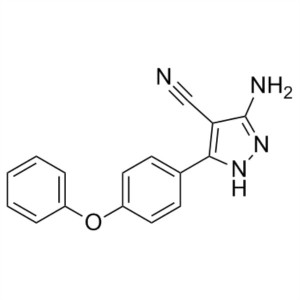
Ibrutinib Intermediate CAS 330792-70-6 Purity >...
-
![4-Amino-3-Iodo-1H-Pyrazolo[3,4-d]pyrimidine CAS 151266-23-8 Ibrutinib Intermediate Purity >98.5% (HPLC)](https://www.ruifuchem.com/uploads/4-Amino-3-Iodo-1H-Pyrazolo34-dpyrimidine-CAS-151266-23-8-Purity-98.5-HPLC-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
4-Amino-3-Iodo-1H-Pyrazolo[3,4-d]pyrimidine CAS...
-
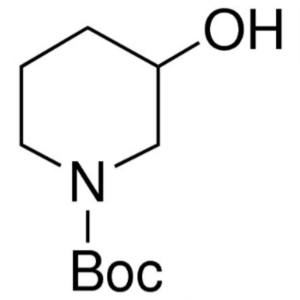
1-Boc-3-Hydroxypiperidine CAS 85275-45-2 Ibruti...
-
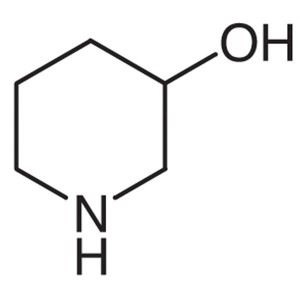
3-Hydroxypiperidine CAS 6859-99-0 Ibrutinib Int...
-
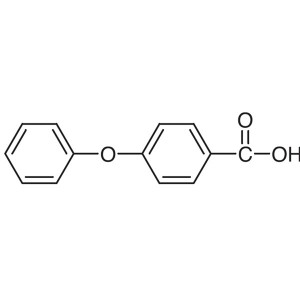
4-Phenoxybenzoic Acid CAS 2215-77-2 Ibrutinib I...
-
![4-Aminopyrazolo[3,4-d]pyrimidine CAS 2380-63-4 Ibrutinib Intermediate Purity >98.0% (HPLC)](https://www.ruifuchem.com/uploads/4-Aminopyrazolo34-dpyrimidine-CAS-2380-63-4-Purity-98.0-HPLC-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
4-Aminopyrazolo[3,4-d]pyrimidine CAS 2380-63-4 ...