Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPLC) API Factory Antiviral High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Acyclovir (CAS: 59277-89-3) with high quality, API, used to treat HSV and VZV Infections. Ruifu Chemical has been supplying pharmaceutical intermediates and APIs more than 15 years. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Acyclovir or other products, please e-mail: alvin@ruifuchem.com
| Chemical Name | Acyclovir |
| Synonyms | ACV; Acycloguanosine; 9-[(2-Hydroxyethoxy)methyl]guanine; Aciclovir |
| CAS Number | 59277-89-3 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C8H11N5O3 |
| Molecular Weight | 225.2 |
| Melting Point | 256.0~257.0℃ |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Item | Specifications | Results |
| Appearance | White Crystalline Powder | Conforms |
| Infrared Absorption | Positive | Conforms |
| Retention Time (HPLC) | Positive | Conforms |
| Water Content (by K.F) | ≤6.00% | 5.20% |
| Ordinary Impurities (TLC) | ≤1.00% | <1.00% |
| Limit for Guanine (HPLC) | ≤0.70% | 0.18% |
| Heavy Metals | ≤20ppm | Conforms |
| Assay / Analysis Method | 98.0%-101.0% (Calculated on the Anhydrous Basis) | 99.20% |
| Conclusion | The product has been tested and complies with the USP35 Standard. | |
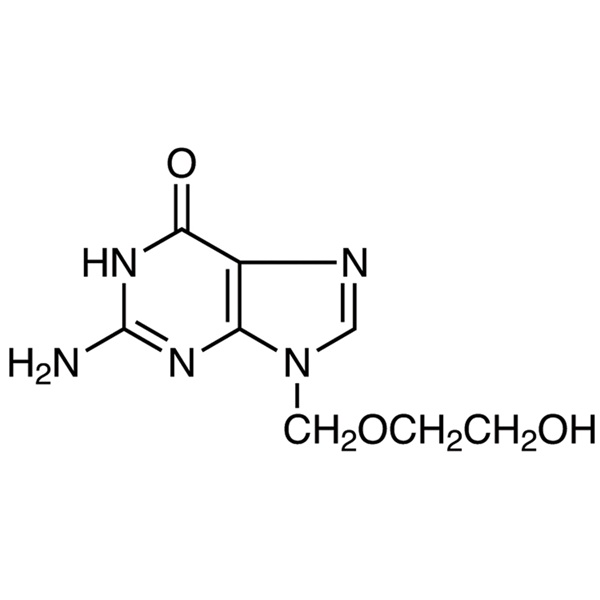
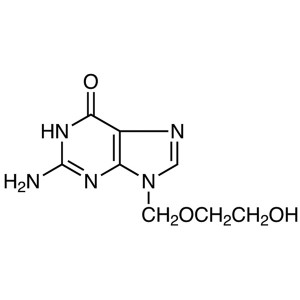
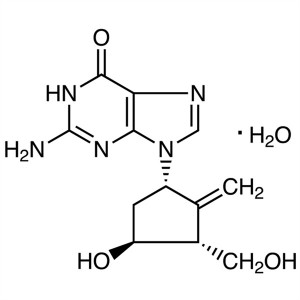
Acyclovir
C8H11N5O3 225.20
6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-.
9-[(2-Hydroxyethoxy)methyl]guanine [59277-89-3].
» Acyclovir contains not less than 98.0 percent and not more than 101.0 percent of C8H11N5O3, calculated on the anhydrous basis.
Packaging and storage- Preserve in tight containers. Store at room temperature. Protect from light and moisture.
USP Reference standards <11>-
USP Acyclovir RS -
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay and limit for guanine.
Water, Method I <921>: not more than 6.0%.
Ordinary impurities <466>-
Test solution: dimethyl sulfoxide.
Standard solution: dimethyl sulfoxide.
Eluant: a mixture of chloroform, methanol, and ammonium hydroxide (80:20:2).
Visualization: 1.
Application volume: 5 µL.
Limit: 1%.
Assay and limit for guanine-
Mobile phase- Prepare a filtered and degassed solution of glacial acetic acid in water (1 in 1000). Make adjustments if necessary (see System Suitability under Chromatography <621>).
System suitability solution 1- Dissolve accurately weighed quantities of USP Acyclovir RS and guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having known concentrations of about 0.1 mg of each per mL.
System suitability solution 2- Dissolve an accurately weighed quantity of guanine in 0.1 N sodium hydroxide, and dilute quantitatively, and stepwise if necessary, with water to obtain a solution having a known concentration of about 0.7 µg per mL.
Guanine standard preparation- Transfer about 8.75 mg of guanine, accurately weighed, to a 500-mL volumetric flask. Dissolve in 50 mL of 0.1 N sodium hydroxide, dilute with water to volume, and mix. Transfer 2.0 mL of this solution to a 50-mL volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix to obtain a solution having a concentration of about 0.7 µg per mL.
Standard preparation- Dissolve about 25 mg of USP Acyclovir RS, accurately weighed, in 5 mL of 0.1 N sodium hydroxide in a 50-mL volumetric flask, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix to obtain a solution having a known concentration of about 0.1 mg of USP Acyclovir RS per mL.
Assay preparation- Dissolve about 100 mg of Acyclovir, accurately weighed, in 20 mL of 0.1 N sodium hydroxide in a 200-mL volumetric flask, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 50-mL volumetric flask, dilute with 0.01 N sodium hydroxide to volume, and mix.
Chromatographic system (see Chromatography <621>)- The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L1. The flow rate is about 3 mL per minute. Chromatograph System suitability solution 1, and record the peak responses as directed for Procedure: the resolution, R, between acyclovir and guanine is not less than 2.0; the tailing factor for the analyte peak is not more than 2; and the relative standard deviation for replicate injections for the acyclovir peak is not more than 2.0%. Chromatograph System suitability solution 2, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure- Separately inject equal volumes (about 20 µL) of the Standard preparation, the Guanine standard preparation, and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for all the peaks. Calculate the quantity, in µg, of guanine in the portion of Acyclovir taken by the formula:
1000C(rU / rS)
in which C is the concentration, in µg per mL, of guanine in the Guanine standard preparation; and rU and rS are the peak responses due to guanine in the Assay preparation and the Guanine standard preparation, respectively: not more than 0.7% of guanine is found. Calculate the quantity, in mg, of C8H11N5O3 in the portion of Acyclovir taken by the formula:
1000C(rU / rS)
in which C is the concentration, in mg per mL, of USP Acyclovir RS in the Standard preparation; and rU and rS are the peak responses due to acyclovir in the Assay preparation and the Standard preparation, respectively.
Package: Bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Risk Codes R36/37/38 - Irritating to eyes, respiratory system and skin.
R40 - Limited evidence of a carcinogenic effect
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
Safety Description S22 - Do not breathe dust.
S24/25 - Avoid contact with skin and eyes.
S36 - Wear suitable protective clothing.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S23 - Do not breathe vapour.
WGK Germany 2
RTECS UP0791400
HS Code 2933990099
Hazard Class IRRITANT
Acyclovir (ACV, CAS 59277-89-3), also known as Acycloguanosine, is an antiviral medication. It is primarily used for the treatment of herpes simplex infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein-Barr virus infection. It can be taken by mouth, applied as a cream, or injected. Acyclovir is used to treat HSV and VZV infections. Acyclovir is a synthetic purine analog derived from guanine. It exerts its effects on the herpes simplex virus (HSV) and varicella-zoster virus by interfering with DNA synthesis through phosphorylation by viral thymidine kinase and subsequent inhibition of viral DNA polymerase, thereby inhibiting viral replication. Acyclovir was patented in 1974, and approved for medical use in 1981. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication and is marketed under many brand names worldwide.