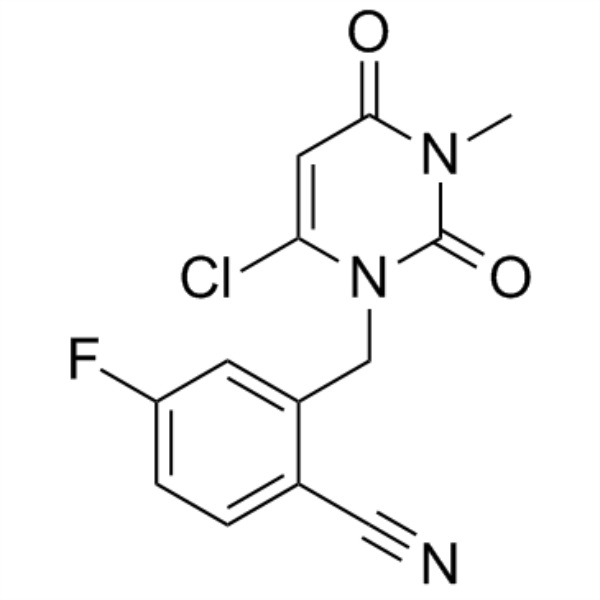
Trelagliptin Succinate Intermediate CAS 865759-24-6 Purity >98.0% (HPLC)
Commercial Supply Trelagliptin Succinate Related Intermediates:
6-Chloro-3-Methyluracil CAS 4318-56-3
2-Cyano-5-Flurobenzyl Bromide CAS 421552-12-7
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 334618-23-4
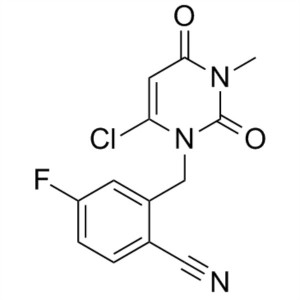
Trelagliptin Succinate Intermediate-int D CAS 865759-24-6
Trelagliptin Succinate CAS 1029877-94-8
Please contact: alvin@ruifuchem.com
| Chemical Name | Trelagliptin Succinate Intermediate-int D |
| Synonyms | 2-((6-Chloro-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)-4-fluorobenzonitrile; 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluorobenzonitrile |
| Impurity | Trelagliptin Impurity 17 |
| Stock Status | In Stock, Commercial Scale |
| CAS Number | 865759-24-6 |
| Molecular Formula | C13H9ClFN3O2 |
| Molecular Weight | 293.68 g/mol |
| Density | 1.49±0.10 g/cm3 |
| Storage Temp. | Cool & Dry Place |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | Off-White to Light Yellow Crystal |
Conforms |
| Identification | ||
| HPLC | The retention time of test sample major peak complies to reference standard | Conforms |
| IR - Infrared Spectrum | IR Absorption spectra of sample and standard are concordant | Conforms |
| Inspection | ||
| Loss on Drying | <0.50% | 0.3% |
| Total Impurities | <2.00% | Conforms |
| Purity / Analysis Method | >98.0% (HPLC) | 99.1% |
| Conclusion | The product has been tested & complies with the specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Avoid light; Airtight.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
2-((6-chloro-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)-4-fluorobenzonitrile (CAS: 865759-24-6) is used as an intermediate of Trelagliptin Succinate (CAS: 1029877-94-8). Trelagliptin Succinate, developed by Takeda, Japan, launched in March 2015, under the trade name Zafatek, is used to treat type 2 diabetes. Trelagliptin is an ultra-long-acting dipeptidyl peptidase IV(DPP-4) inhibitor by selectively and continuously inhibiting DPP-4 to control blood sugar levels. Trelagliptin is the first weekly hypoglycemic drug on the market, and similar DPP-4 inhibitors on the market need to be taken once a day. Zafatek medication advantages will undoubtedly provide more convenient treatment options for diabetic patients. It is expected to greatly improve the convenience and compliance of patients. on March 26, 2015, Japanese pharmaceutical giant Takeda announced that the new diabetes drug Zafatek was approved by Japan's Ministry of health, labor and welfare (MHLW) for the treatment of type 2 diabetes. The approval marks Zafatek becoming the first weekly oral hypoglycemic drug on the market in the world, and also represents a blockbuster dropped by Takeda in the diabetes market.
Trelagliptin is a once-weekly dipeptidyl peptidase IV (DPP-4) inhibitor that controls blood sugar levels by selectively and continuously inhibiting DPP-4. DPP-4 is an enzyme that can initiate the inactivation of incretin (glucagon-like peptide -1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)), which play an important role in blood glucose regulation. Inhibition of DPP-4 can increase blood sugar level-dependent insulin secretion, thus controlling blood sugar level. Trelagliptin NDA submission is based on efficacy and safety data from several phase III clinical trials conducted in Japanese patients with type 2 diabetes. The efficacy of Trelagliptin has been confirmed in all trials, and it has good safety and tolerability. Trelagliptin Administration once a week can effectively control blood sugar level and is expected to improve patients' medication compliance.
-
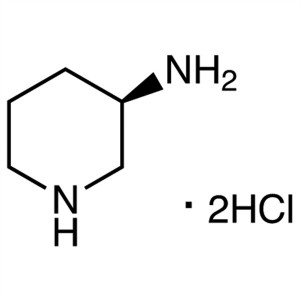
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
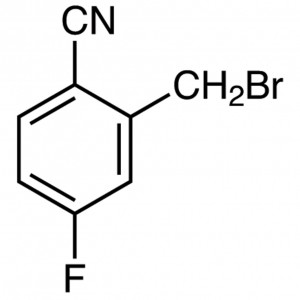
2-Cyano-5-Flurobenzyl Bromide CAS 421552-12-7 P...
-
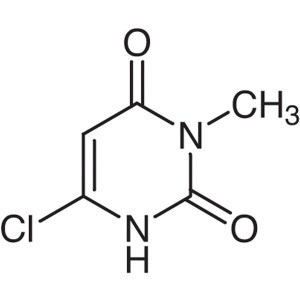
6-Chloro-3-Methyluracil CAS 4318-56-3 Purity ≥9...
-
Trelagliptin Succinate Intermediate CAS 865759-...
-
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3 Lin...
-
2-(Chloromethyl)-4-Methylquinazoline CAS 109113...
-
1-Bromo-2-Butyne CAS 3355-28-0 Purity ≥99.0% (G...
-
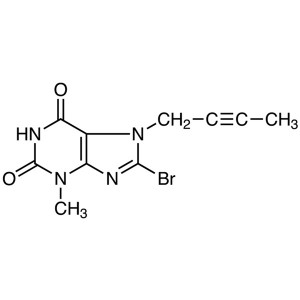
8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine CAS 6...
-
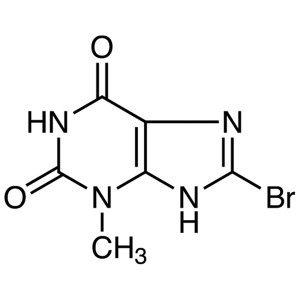
8-Bromo-3-Methylxanthine CAS 93703-24-3 Linagli...
-
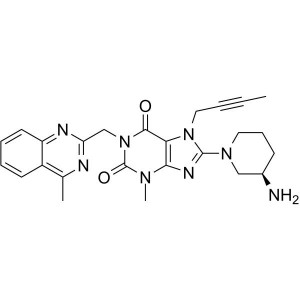
Linagliptin CAS 668270-12-0 Purity ≥99.0% (HPLC...
-
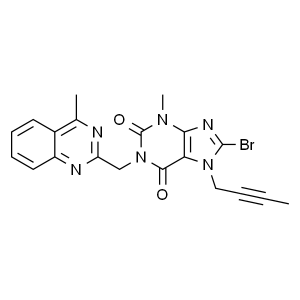
Linagliptin Parent Nucleus Intermediate CAS 853...