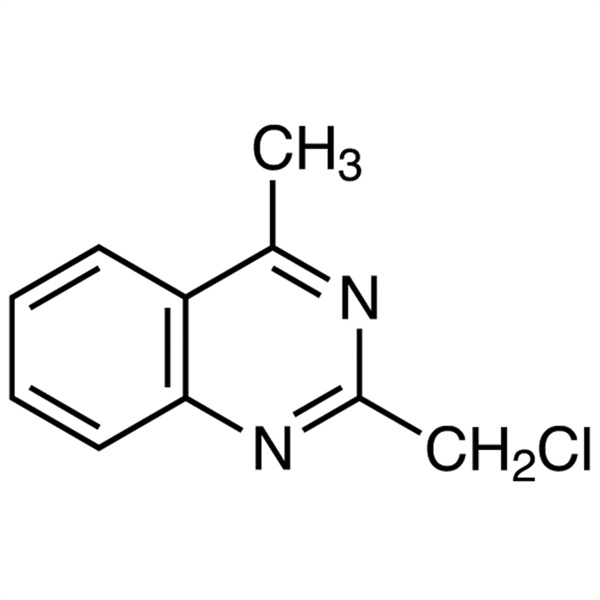
2-(Chloromethyl)-4-Methylquinazoline CAS 109113-72-6 Linagliptin Intermediate Purity ≥99.0% (HPLC)
Manufacturer Supply Linagliptin and Related Intermediates:
Linagliptin CAS 668270-12-0
Linagliptin Parent Nucleus Intermediate CAS 853029-57-9
8-Bromo-3-Methylxanthine CAS 93703-24-3
8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine CAS 666816-98-4
2-(Chloromethyl)-4-Methylquinazoline CAS 109113-72-6
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 334618-23-4
1-Bromo-2-Butyne CAS 3355-28-0
| Chemical Name | 2-(Chloromethyl)-4-Methylquinazoline |
| Synonyms | Linagliptin Intermediate A |
| CAS Number | 109113-72-6 |
| CAT Number | RF-PI498 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H9ClN2 |
| Molecular Weight | 192.64 |
| Density | 1.251 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Pale Yellow to Pale Brown Powder |
| Identification A | The sample IR spectrum must match with the standard spectrum |
| Identification B | The retention time of principle peak of the sample must correspond to standard |
| Solubility | Soluble in Methylene Chloride; Insoluble in Water |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Melting Point | 60.0 to 65.0℃ |
| Water (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.50% |
| Related Substances | |
| Single Impurity | ≤0.30% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate for Linagliptin (CAS: 668270-12-0) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


2-(Chloromethyl)-4-Methylquinazoline (CAS: 109113-72-6) is used as an intermediate for the preparation of Linagliptin (CAS: 668270-12-0), and its impurities. Linagliptin, sold under the brand name Tradjenta among others, is a medication used to treat diabetes mellitus type 2. It is generally less preferred than metformin and sulfonylureas as an initial treatment. It is used together with exercise and diet. It is not recommended in type 1 diabetes. It works by increasing the production of insulin and decreasing the production of glucagon by the pancreas. Linagliptin was approved for medical use in the United States in 2011. Linagliptin is a highly potent, selective dipeptidyl peptidase-4 (DPP-4) inhibitor with IC50 of 1 nM.
-
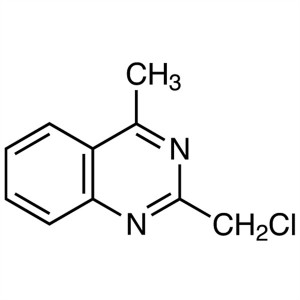
2-(Chloromethyl)-4-Methylquinazoline CAS 109113...
-
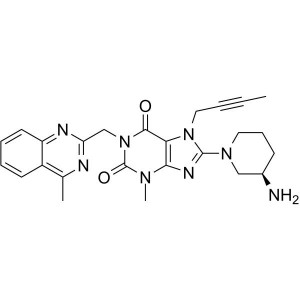
Linagliptin CAS 668270-12-0 Purity ≥99.0% (HPLC...
-
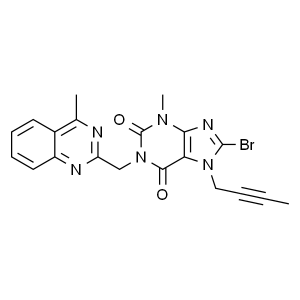
Linagliptin Parent Nucleus Intermediate CAS 853...
-
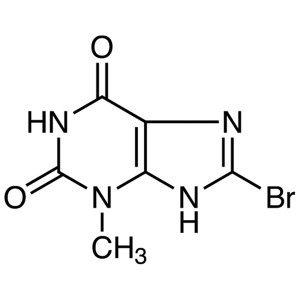
8-Bromo-3-Methylxanthine CAS 93703-24-3 Linagli...
-
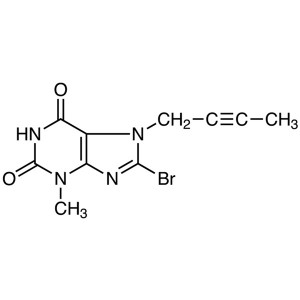
8-Bromo-7-(2-butyn-1-yl)-3-methylxanthine CAS 6...
-
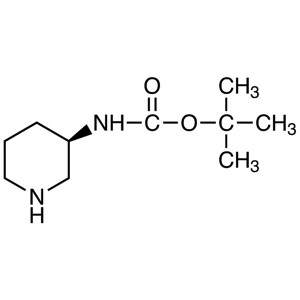
(R)-3-(Boc-Amino)piperidine CAS 309956-78-3 Lin...
-
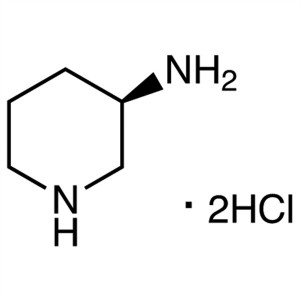
(R)-(-)-3-Aminopiperidine Dihydrochloride CAS 3...
-
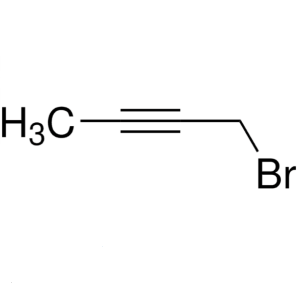
1-Bromo-2-Butyne CAS 3355-28-0 Purity ≥99.0% (G...