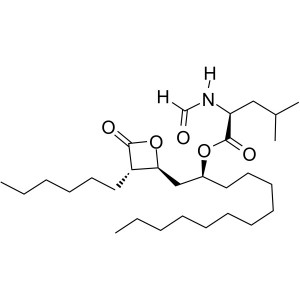
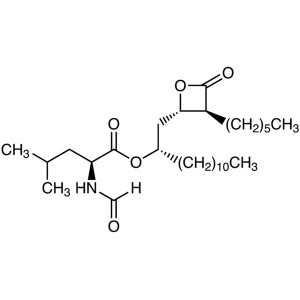
Orlistat CAS 96829-58-2 API Weight Loss Drug Purity 98.0~101.5%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Orlistat (CAS: 96829-58-2) with high quality, commercial production, weight loss drug. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Orlistat, Please contact: alvin@ruifuchem.com
| Chemical Name | Orlistat |
| Synonyms | N-Formyl-L-Leucine (1S)-1-[[(2S,3S)-3-Hexyl-4-Oxo-2-Oxetanyl]Methyl] Dodecyl Ester; (S)-2-Formylamino-4-Methyl-Pentanoic Acid (S)-1-[[(2S,3S)-3-Hexyl-4-Oxo-2-Oxetanyl]Methyl]-Dodecyl Ester ; Tetrahydrolipstatin; Ro-18-0647 |
| CAS Number | 96829-58-2 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C29H53NO5 |
| Molecular Weight | 495.75 |
| Melting Point | 43.0℃~48.0℃ |
| Density | 0.976±0.06 g/cm3 |
| Sensitive | Heat Sensitive |
| Solubility | Soluble in Chloroform |
| Shipping Condition | Under Ambient Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Almost White Crystalline Powder |
| Identification A | Infrared Absorption |
| Identification B | The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. |
| Purity / Analysis Method | 98.0~101.5% of C29H53NO5, calculated on the anhydrous, solvent-free basis |
| Specific Rotation | -48.0° ~ -51.0° |
| Water Determination | ≤0.20% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Orlistat Related Compound A | ≤0.20% |
| Orlistat Related Compound B | ≤0.05% |
| Formylleucinea | ≤0.20% |
| Orlistat Related Compound C | ≤0.05% |
| Orlistat Open Ring Epimer | ≤0.20% |
| D-Leucine Orlistat | ≤0.20% |
| Individual Unidentified Impurity | ≤0.10% |
| Orlistat Related Compound D | ≤0.20% |
| Orlistat Open Ring Amide | ≤0.10% |
| Orlistat Related Compound E | ≤0.20% |
| Total Impurities | ≤1.00% |
| Test Standard | Chinese Pharmacopoeia; USP35 |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Stay away from strong light and heat, moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Orlistat is an internationally recognized new form of weight loss drug. Its commercial name is Sainike and first went on sale in New Zealand in 1998. Orlistat is a long-term and highly effective specific gastrointestinal lipase inhibitor, and it is insoluble in water, soluble in chloroform, and easily soluble in ethanol. Orlistat can be used clinically to treat obesity. Usually, a dose of 120mg is taken three times a day within one hour of a meal. Weight loss begins to occur after two weeks of usage. It can be used continuously for 6-12 months, and its effects will cease to increase after daily dosage exceeds 400mg. This drug is suitable to be used in combination with a low-calorie diet by obese and overweight individuals, and it can also be used as long-term treatment for patients who have faced weight-related risk factors. Orlistat has a long-term weight-control effect that reduces and maintains weight and prevents against rebounding. Using Orlistat can lower the occurrences of weight-related risk factors and diseases, including hypercholesterolemia, type-2 diabetes, impaired glucose tolerance, hyperinsulinemia, and hypertension, and it can reduce the fat content in organs. Orlistat also adjusts blood lipid levels: it can decrease serum triglycerides (TG) and low density lipoprotein cholesterol (LDL-C), and it can increase the ratio of high density lipoproteins to low density lipoproteins in obese patients.
Orlistat
C29H53NO5 495.73
l-Leucine, N-formyl-, 1-[(3-hexyl-4-oxo-2-oxetanyl)methyl]dodecyl ester, [2S-[2(R*), 3]]-;
N-Formyl-l-leucine, ester with (3S,4S)-3-hexyl-4-[(2S)-2-hydroxytridecyl]-2-oxetanone [96829-58-2].
DEFINITION
Orlistat contains NLT 98.0% and NMT 101.5% of C29H53NO5, calculated on the anhydrous, solvent-free basis.
IDENTIFICATION
• A. Infrared Absorption <197M>
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note-Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis. ]
Mobile phase: Acetonitrile, phosphoric acid, and water (860: 0.05: 140)
Standard solution: 0.5 mg/mL of USP Orlistat RS in Mobile phase. Inject immediately after preparation or store at 5.
Sample solution: 0.5 mg/mL of Orlistat in Mobile phase. Inject immediately after preparation or store at 5.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 195
Column: 3.9-mm × 15-cm; 4-µm packing L1
Flow rate: 1.0 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of orlistat (C29H53NO5) in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-101.5% on the anhydrous, solvent-free basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.1%
• Heavy Metals, Method II <231>: 20 ppm
Organic Impurities
• Procedure 1: Limit of Orlistat Related Compound A
Standard solution: 0.1 mg/mL of USP Orlistat Related Compound A RS in acetone
Sample solution: 50 mg/mL of Orlistat in acetone
Chromatographic system
(See Chromatography <621>, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Toluene and ethyl acetate (4:1)
Detection solution: Transfer 2.5 g of phosphomolybdic acid and 1 g of cerric sulfate into a 100-mL volumetric flask, dissolve in and dilute with methanol to volume.
Analysis
Samples: Standard solution and Sample solution
Remove the plate, and air-dry it thoroughly. Spray the dried plate with Detection solution, and place the plate in an oven at 120 for 30 min.
Acceptance criteria: Any secondary spot from the Sample solution corresponding to orlistat related compound A is not more intense than the corresponding spot from the Standard solution (0.2%).
• Procedure 2: Limit of Orlistat Related Compound B
Standard solution: 0.025 mg/mL of USP Orlistat Related Compound B RS in methylene chloride
Sample solution: 50 mg/mL of Orlistat in methylene chloride
Spiked sample solution: 50 mg/mL of Orlistat in Standard solution
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm x 30-m fused silica, coated with a 0.25-µm G27 stationary phase
Column temperature: See the temperature program table below.
Initial Temperature () Temperature Ramp (/min) Final Temperature () Hold Time at Final Temperature (min)
50 4 170 -
170 30 300 30
Temperature
Injector: 270
Detector: 280
Carrier gas: Helium
Flow rate: 30 mL/min
Split ratio: 10:1
Injection size: 2 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 10.0%
Analysis
Samples: Sample solution and Spiked sample solution
Calculate the percentage of orlistat related compound B in the portion of Orlistat taken:
Result = [rU/(rSP rU)] × (CS/CT) × 100
rU = peak response of orlistat related compound B from the Sample solution
rSP = peak response of orlistat related compound B from the Spiked sample solution
CS = concentration of USP Orlistat Related Compound B RS in the Standard solution (mg/mL)
CT = concentration of Orlistat in the Spiked sample solution (mg/mL)
Acceptance criteria: NMT 0.05% of orlistat related compound B is found.
• Procedure 3
[Note-Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis. ]
Mobile phase, Standard solution, and Sample solution: Prepare as directed in the Assay.
System suitability solution: 10 µg/mL of USP Orlistat RS, 0.1 µg/mL of USP Orlistat Related Compound C RS, and 0.25 µg/mL of USP Orlistat Related Compound D RS in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Proceed as directed in the Assay, except to chromatograph the System suitability solution.
System suitability
Sample: System suitability solution
Suitability requirements
Signal-to-noise ratio: NLT 3 for the orlistat related compound C and orlistat related compound D peaks
Relative standard deviation: NMT 10.0% for the orlistat peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response for each individual impurity from the Sample solution
rS = peak response of USP Orlistat RS from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
F = relative response factor as given in Impurity Table 1
Acceptance criteria: See Impurity Table 1.
Impurity Table 1
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Formylleucinea 0.10 4.0 0.2
Orlistat related compound C 0.13 33 0.05
Orlistat open ring epimerb 0.44 1.0 0.2
Orlistat related compound D* 0.90 - Calculated in
Procedure 4
Orlistat open ring amidec* 0.90 - Calculated in
Procedure 4
Orlistat 1.00 - -
d-Leucine orlistatd 1.18 1.0 0.2
Individual unidentified impurity - 1.0 0.1
* Coelutes in this LC system, determined using Procedure 4.
a N-Formyl-l-leucine.
b (2S,3R,5S)-5-[(S)-2-Formylamino-4-methyl-pentanoyloxy]-2-hexyl-3-hydroxy-hexadecanoic acid.
c N-Formyl-l-leucine (S)-1-[(2S,3S)-2-hydroxy-3-[1-phenyl-R-ethylcarbomoyl]nonyl]-dodecyl ester.
d N-Formyl-d-leucine (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester or enantiomer.
• Procedure 4: Limit of Orlistat Related Compound D
Mobile phase: Methanol and water (83:17)
System suitability solution: 4 mg/mL of USP Orlistat RS and 2.4 µg/mL of USP Orlistat Related Compound D RS in acetonitrile, respectively
Standard solution: 5.0 mg/mL of USP Orlistat RS in acetonitrile
Sample solution: 5.0 mg/mL of Orlistat in acetonitrile
Chromatographic system
(See Chromatography 621, System Suitability.)
Mode: LC
Detector: 205 nm
Column: 4.0-mm × 25-cm; 5-µm packing L7
Flow rate: 0.6 mL/min
Injection size: 20 µL
System suitability
Sample: System suitability solution
Suitability requirements
Signal-to-noise ratio: NLT 3 for the orlistat related compound D peak
Relative standard deviation: NMT 10.0% for the orlistat peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response for each individual impurity from the Sample solution
rS = peak response for USP Orlistat RS from the Standard solution
CS = concentration of USP Orlistat RS in the Standard solution (µg/mL)
CU = concentration of Orlistat in the Sample solution (µg/mL)
F = relative response factor as obtained in Impurity Table 2
Acceptance criteria: See Impurity Table 2.
Impurity Table 2
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Orlistat related compound D 0.94 1.0 0.2
Orlistat 1.00 - -
Orlistat open ring amidea 1.25 4.3 0.1
a N-Formyl-l-leucine (S)-1-[(2S,3S)-2-hydroxy-3-[1-phenyl-R-ethylcarbomoyl]nonyl]-dodecyl ester.
• Procedure 5: Limit of Orlistat Related Compound E
Buffer: 0.4 N borate solution, adjusted to a pH of 10.2
Derivitizing agent: o-Phthaldehyde (OPA) solution. [Note-If unable to obtain commercially, the Derivitizing agent can be prepared as 1% each of 3-mercaptopropionic acid and o-phthaldialdehyde in 0.4 M borate buffer solution.]
Solution A: Transfer 4.1 g of sodium acetate trihydrate and 40 mg of ethylenediaminetetraacetic acid (EDTA) into a 1-L volumetric flask. Dissolve in 950 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Dilute with water to volume, add 2.5 mL of tetrahydrofuran, and mix. Filter, and degas.
Solution B: Transfer 2.7 g of sodium acetate trihydrate and 40 mg of EDTA into a 1-L volumetric flask. Dissolve in 200 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Add 800 mL of acetonitrile, filter, and degas.
Mobile phase: See the gradient table below.
Time (min) Solution A (%) Solution B (%)
0 96.7 3.3
20 60 40
24 0 100
38 0 100
38 96.7 3.3
45 96.7 3.3
Standard solution: Transfer a weighed quantity of about 0.2 mg of USP Orlistat Related compound E RS into a 20-mL head-space vial. Add 10 mL of 4 N sodium hydroxide, and close the vial. Heat the vial to 100 for 1 h, then allow to cool to room temperature. Transfer 2 mL of the resulting solution into a 50-mL volumetric flask, and dilute with water to volume. To 0.5 mL of this solution add 2.0 mL of Buffer and 0.5 mL of Derivatizing agent.
Sample solution: Proceed as directed for the Standard solution, but instead use 25 mg of Orlistat to replace the 0.2 mg of USP Orlistat Related Compound E RS.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: Fluorescence 340 nm (excitation); 450 nm (emission)
Columns
Guard: 2.1-mm × 2-cm; 50-µm packing L1
Analytical: 2.1-mm × 20-cm; packing L1
Flow rate: 0.5 mL/min
Injection size: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 6.0% for the orlistat related compound E peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of this impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response for orlistat related compound E in the Sample solution
rS = peak response for USP Orlistat Related Compound E RS in the Standard solution
CS = concentration of USP Orlistat Related Compound E RS in the Standard solution (mg/mL)
CU = concentration of Orlistat in the Sample solution (mg/mL)
Acceptance criteria
Individual impurity: NMT 0.2% of orlistat related compound E is found.
Total impurities: NMT 1.0% of total impurities is found, the results for Procedures 1, 2, 3, 4, and 5 being added.
SPECIFIC TESTS
• Optical Rotation, Specific Rotation <781>
Sample solution: 30 mg/mL in dehydrated alcohol
Acceptance criteria: Between -48.0 and -51.0, at 20
• Water Determination, Method Ic <921>: NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in well-closed containers between 2 and 8℃.
• USP Reference Standards <11>
USP Orlistat RS
USP Orlistat Related Compound A RS
USP Orlistat Related Compound B RS
USP Orlistat Related Compound C RS
USP Orlistat Related Compound D RS
USP Orlistat Related Compound E RS
USP35
-
Orlistat CAS 96829-58-2 API Weight Loss Drug Pu...
-
Ethyl 4-Aminobenzoate (Benzocaine) CAS 94-09-7 ...
-
Baloxavir Marboxil CAS 1985606-14-1 API Factory...
-
Azithromycin Dihydrate CAS 117772-70-0 Assay 94...
-
Azelastine Hydrochloride CAS 79307-93-0 Assay 9...
-
Acotiamide Hydrochloride Trihydrate CAS 773092-...
-
Abacavir Sulfate CAS 188062-50-2 Assay 98.0%~10...
-
Abacavir CAS 136470-78-5 Assay 99.0%~101.0% API...
-
Vildagliptin CAS 274901-16-5 Purity ≥99.0% (HPL...
-
Sorafenib Tosylate CAS 475207-59-1 Purity ≥99.0...
-
Vonoprazan Fumarate (TAK-438) CAS 1260141-27-2 ...
-
Acyclovir CAS 59277-89-3 Assay 98.0-101.0% (HPL...
-
Argatroban CAS 74863-84-6 API Factory High Puri...
-
Anastrozole CAS 120511-73-1 API Factory High Qu...
-
Argatroban Monohydrate CAS 141396-28-3 Purity ≥...
-
Atazanavir CAS 198904-31-3 Purity ≥99.0% API Fa...