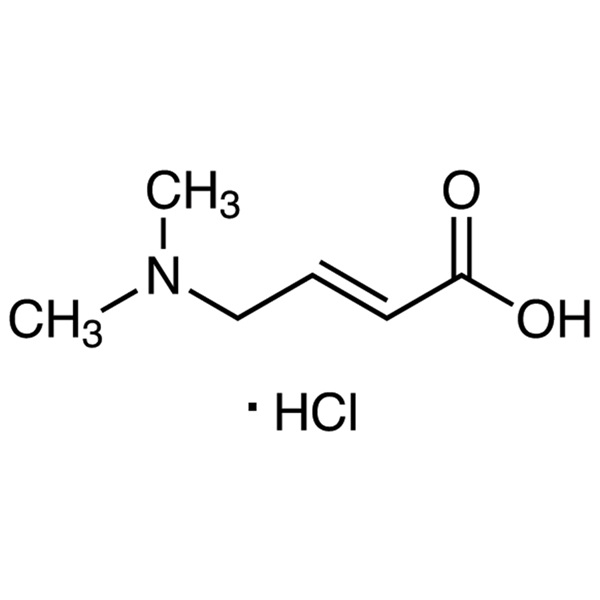
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7 Purity >98.0% (HPLC) Afatinib Dimaleate Intermediate
Ruifu Chemical Supply Intermediates of Afatinib
Afatinib CAS 439081-18-2
Afatinib Dimaleate CAS 850140-73-7
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7
Diethylphosphonoacetic Acid CAS 3095-95-2
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012-69-3
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449-14-2
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1
(S)-N4-(3-Chloro-4-Fluorophenyl)-7-((Tetrahydrofuran-3-yl)oxy)quinazoline-4,6-Diamine CAS 314771-76-1
(S)-N-(3-Chloro-4-Fluorophenyl)-6-Nitro-7-((Tetrahydrofuran-3-yl)oxy)quinazolin-4-Amine CAS 314771-88-5
| Chemical Name | trans-4-Dimethylaminocrotonic Acid Hydrochloride |
| Synonyms | trans 4-Dimethylaminocrotonic Acid HCl; (E)-4-(Dimethylamino)-2-Butenoic Acid Hydrochloride; (E)-4-Dimethylaminocrotonic Acid Hydrochloride; (2E)-4-(Dimethylamino)but-2-Enoic Acid Hydrochloride; Afatinib int-2 |
| CAS Number | 848133-35-7 |
| Stock Status | In Stock, Commercial Scale |
| Molecular Formula | C6H12ClNO2 |
| Molecular Weight | 165.62 |
| Sensitivity | Hygroscopic. Moisture Sensitive |
| Melting Point | 160.0 to 164.0℃ |
| Solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Almost White Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Purity / Analysis Method | >98.0% (NMR) |
| Moisture (K.F) | <0.50% |
| Residue on Ignition | <0.20% |
| Single Impurity | <0.50% |
| Heavy Metals (as Pb) | <20ppm |
| Infrared Spectrum | Conforms to Structure |
| 1 H NMR Spectrum | Proton NMR Spectrum |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Afatinib, Afatinib Dimaleate |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Instrument: Agilent 1200 HPLC HPLC chromatograph, DAD detector.
Column: Agilent XDB-C18,250*4.6mm, 5μm
Mobile phase: B: 1.95g sodium octane sulfonate + 8ml phosphoric acid + 5ml triethylamine + 500ml water
C: acetonitrile
Preparation of mixed mobile phase: take 400ml B solution and 100ml acetonitrile, mix thoroughly and evenly, and pump the liquid in one phase.
Flow rate: 0.5ml/min54bar
Column temperature: 25℃
Wavelength: 210nm
Sample solution: mobile phase was used as solvent, solid sample: 0.0040g/2ml, sample size 2.0μl.

trans-4-Dimethylaminocrotonic Acid Hydrochloride (CAS: 848133-35-7) is a reagent used in the preparation of tyrosine kinase inhibiting antitumor agents. trans-4-Dimethylaminocrotonic Acid Hydrochloride can be used as an intermediate of Afatinib (CAS: 439081-18-2), Afatinib Dimaleate (CAS: 850140-73-7), Neratinib (CAS: 698387-09-6). Afatinib is a drug approved for the treatment non-small cell lung carcinoma (NSCLC), developed by Boehringer Ingelheim. It acts as a angiokinase inhibitor. Like Lapatinib and Neratinib, Afatinib is a tyrosine kinase inhibitor (TKI) that also irreversibly inhibits human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) kinases. Afatinib is not only active against EGFR mutations targeted by first generation TKIs likeerlotinib or gefitinib, but also against those not sensitive to these standard therapies. Because of its additional activity against Her2, it is being investigated for breast cancer as well as other EGFR and Her2 driven cancers. Neratinib developed by US Wyeth company is an irreversible epidermal growth factor receptor(EGFR) inhibitor. It is a multiple target point of small molecule tyrosine kinase inhibitors to HER 2 and HER1 after Lapatinib, and is an irreversible ErbB receptor tyrosine kinase inhibitor. Neratinib could selectively inhibit HER-1 and HER-2 of EGFR family(IC50 was 92 nmol/L and 59 nmol/L, respectively). Clinical research showed that Neratinib exerted significant therapeutic effect on non-small cell lung cancer, colon cancer, and breast cancer. The phaseⅡclinical trial indicated that Neratinib showed good efficacy and tolerance to HER-2 positive patients with advanced breast cancer who had been received or not Trastuzumab treatment. The phase Ⅲ breast cancer clinical trial was complete in September 2014.
-
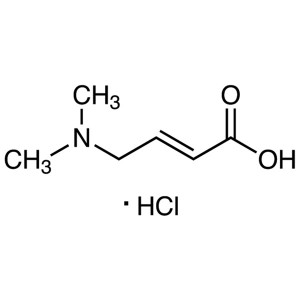
trans-4-Dimethylaminocrotonic Acid Hydrochlorid...
-
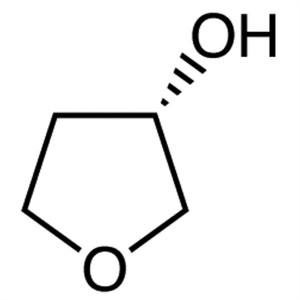
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2...
-
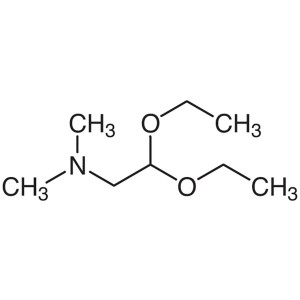
(Dimethylamino)acetaldehyde Diethyl Acetal CAS ...
-
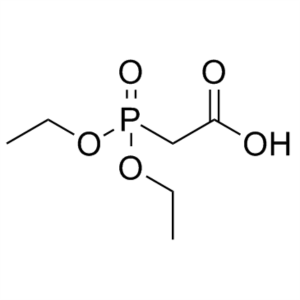
Diethylphosphonoacetic Acid CAS 3095-95-2 Purit...
-
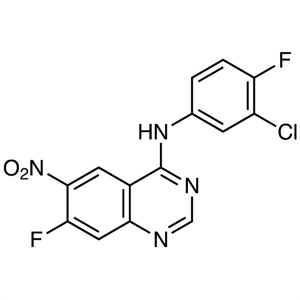
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroqui...
-
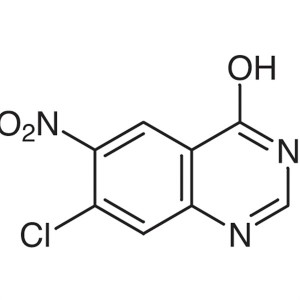
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449...
-
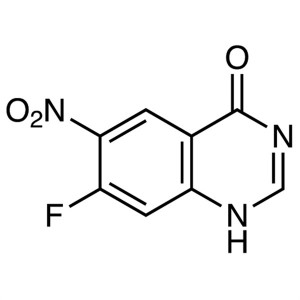
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012...
-
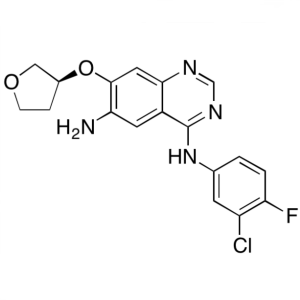
Afatinib Dimaleate Intermediate CAS 314771-76-1...
-
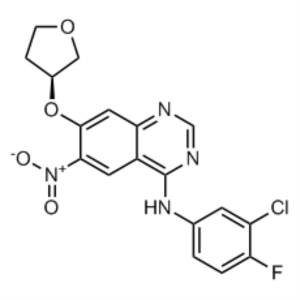
Afatinib Dimaleate Intermediate CAS 314771-88-5...
-
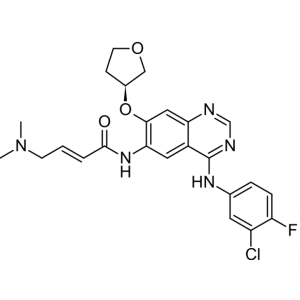
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) F...
-
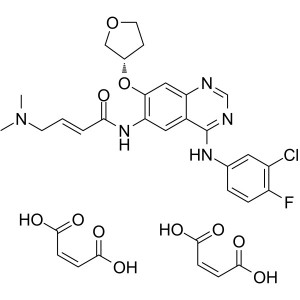
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...