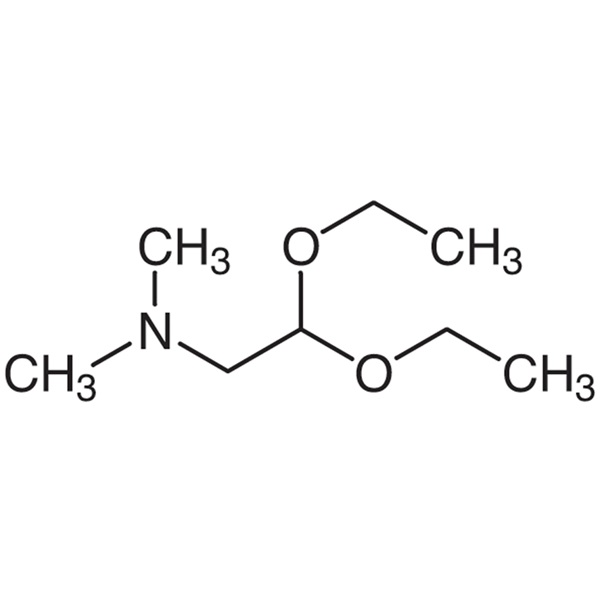
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6 Purity >99.0% (GC) Afatinib Dimaleate Intermediate Factory
Ruifu Chemical Supply Intermediates of Afatinib
Afatinib CAS 439081-18-2
Afatinib Dimaleate CAS 850140-73-7
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7
Diethylphosphonoacetic Acid CAS 3095-95-2
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012-69-3
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449-14-2
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1
(S)-N4-(3-Chloro-4-Fluorophenyl)-7-((Tetrahydrofuran-3-yl)oxy)quinazoline-4,6-Diamine CAS 314771-76-1
(S)-N-(3-Chloro-4-Fluorophenyl)-6-Nitro-7-((Tetrahydrofuran-3-yl)oxy)quinazolin-4-Amine CAS 314771-88-5
| Chemical Name | (Dimethylamino)acetaldehyde Diethyl Acetal |
| Synonyms | 2,2-Diethoxy-N,N-Dimethylethylamine; N-(2,2-Diethoxyethyl)dimethylamine; 2,2-Diethoxy-N,N-Dimethylethan-1-Amine |
| CAS Number | 3616-56-6 |
| CAT Number | RF-PI2031 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C8H19NO2 |
| Molecular Weight | 161.25 |
| Sensitivity | Moisture Sensitive |
| Boiling Point | 60℃/5 mmHg |
| Flash Point | 45℃ |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless Clear Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Refractive Index N20/D | 1.410~1.414 |
| Specific Gravity (20/20℃) | 0.865~0.870 |
| Proton NMR Spectrum | Conforms to Structure |
| Total Impurities | <1.00% |
| Heavy Metals (as Pb) | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates; Afatinib Dimaleate; Sumatriptan |
Package: Fluorinated Bottle, 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(Dimethylamino)acetaldehyde Diethyl Acetal (CAS: 3616-56-6) is used as pharmaceutical intermediates. (Dimethylamino)acetaldehyde Diethyl Acetal can be used in the synthesis of Afatinib Dimaleate (CAS: 850140-73-7), Sumatriptan (CAS: 103628-46-2). Afatinib (trade name Gilotrif in the US and Giotrif in Europe, previously Tomtovok and Tovok) is a drug approved in United States, Europe, Taiwan, Mexico, Chile and Japan as well as other countries for the first-line treatment of patients with distinct types of metastatic (EGFR mutation positive) non-small cell lung carcinoma (NSCLC), developed by Boehringer Ingelheim. Afatinib acts as an irreversible covalent inhibitor of thereceptor tyrosine kinases epidermal growth factor receptor (EGFR) and erbB-2 (HER2). Sumatriptan was the first triptan approved (1991) for theacute treatment of migraine headaches. It has the lowestoral bioavailability among all triptans because of its lowlipophilicity. The availability of many different dosageforms (i.e., an oral tablet, a SC injection, a nasal spray formulation,and a suppository) allows the flexibility of tailoringtherapy to the needs of the individual patients, thusmaking sumatriptans a very useful drug for an acute treatmentof migraine headaches. Sumatriptan also has a very fastonset of action via SC injection or nasal spray administration.
-
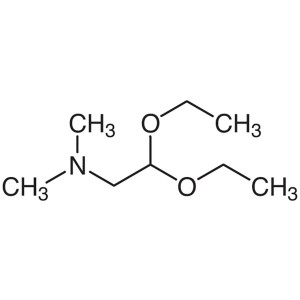
(Dimethylamino)acetaldehyde Diethyl Acetal CAS ...
-
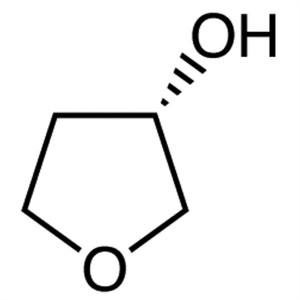
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2...
-
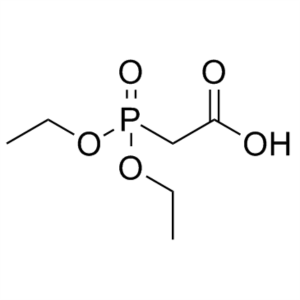
Diethylphosphonoacetic Acid CAS 3095-95-2 Purit...
-
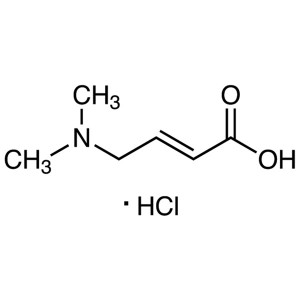
trans-4-Dimethylaminocrotonic Acid Hydrochlorid...
-
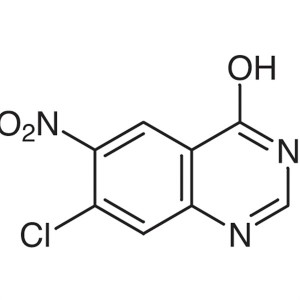
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449...
-
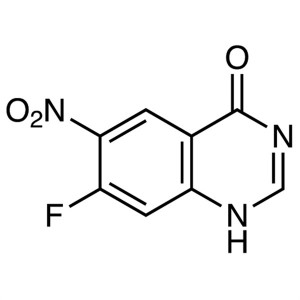
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012...
-
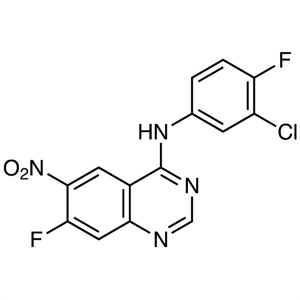
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroqui...
-
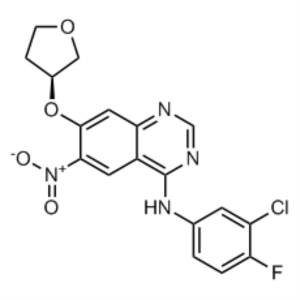
Afatinib Dimaleate Intermediate CAS 314771-88-5...
-
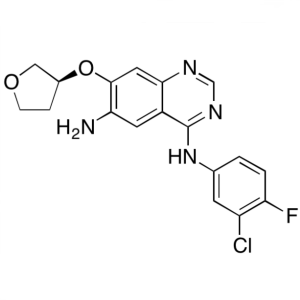
Afatinib Dimaleate Intermediate CAS 314771-76-1...
-
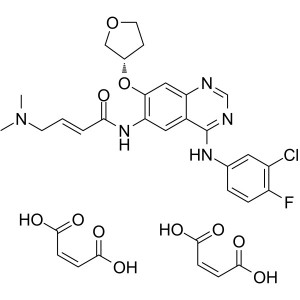
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...
-
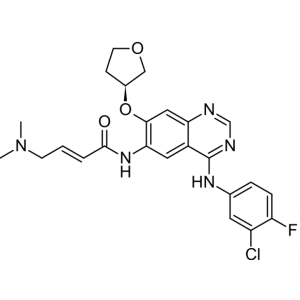
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) F...