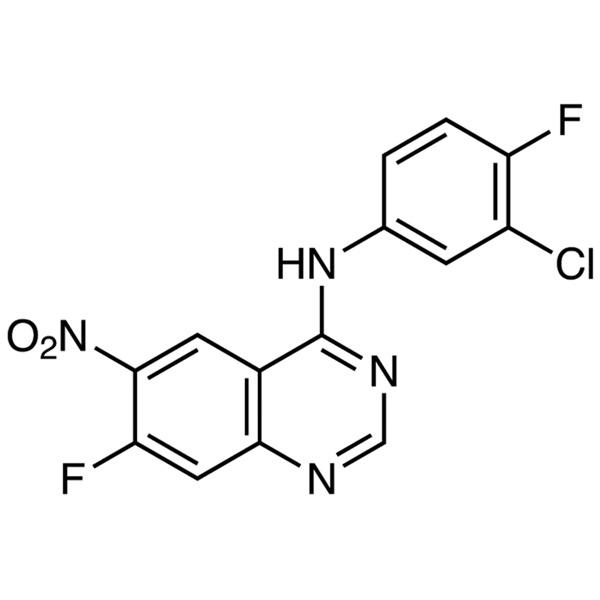
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1 Purity >99.0% (HPLC) Afatinib Dimaleate Intermediate Factory
Ruifu Chemical Supply Intermediates of Afatinib
Afatinib CAS 439081-18-2
Afatinib Dimaleate CAS 850140-73-7
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2
(Dimethylamino)acetaldehyde Diethyl Acetal CAS 3616-56-6
trans-4-Dimethylaminocrotonic Acid Hydrochloride CAS 848133-35-7
Diethylphosphonoacetic Acid CAS 3095-95-2
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012-69-3
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449-14-2
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine CAS 162012-67-1
(S)-N4-(3-Chloro-4-Fluorophenyl)-7-((Tetrahydrofuran-3-yl)oxy)quinazoline-4,6-Diamine CAS 314771-76-1
(S)-N-(3-Chloro-4-Fluorophenyl)-6-Nitro-7-((Tetrahydrofuran-3-yl)oxy)quinazolin-4-Amine CAS 314771-88-5
| Chemical Name | N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine |
| Synonyms | N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitro-4-Quinazolinamine; (3-Chloro-4-Fluorophenyl)(7-Fluoro-6-Nitroquinazolin-4-yl)amine; 4-(3-Chloro-4-Fluoroanilino)-7-Fluoro-6-Nitroquinazoline; Afatinib Intermediate A |
| CAS Number | 162012-67-1 |
| CAT Number | RF-PI2026 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C14H7ClF2N4O2 |
| Molecular Weight | 336.68 |
| Sensitivity | Air Sensitive, Heat Sensitive |
| Melting Point | 242.0~244.0℃ |
| Boiling Point | 489℃ |
| Density | 1.616 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow to Yellow Powder |
| Purity / Analysis Method | >99.0% (HPLC) |
| Loss on Drying | <0.50% |
| Residue on Ignition | <0.20% |
| Related Substances | |
| Individual Impurity | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals | ≤20ppm |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Afatinib Dimaleate (CAS: 850140-73-7) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroquinazolin-4-Amine (CAS: 162012-67-1) is an intermediate in the synthesis of Afatinib Dimaleate (CAS: 850140-73-7). Afatinib Dimaleate is a salt form of Afatinib. Afatinib is a second-generation, orally administered, irreversible inhibitor of the ErbB family of tyrosine kinases. Mechanism of ActionAfatinib downregulates ErbB signalling by covalently binding to the kinase domains of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER) 2 and HER4, resulting in irreversible inhibition of tyrosine kinase autophosphorylation; it also inhibits transphosphorylation of HER3. Afatinib is approved as monotherapy for the treatment of EGFR tyrosine kinase inhibitor (TKI)-naive adults with locally advanced or DefinitionChEBI: A maleate salt obtained by combining afatinib with two molar equivalents of maleic acid. Used for the first-line treatment of patients with metastatic non-small cell lung cancer.
-
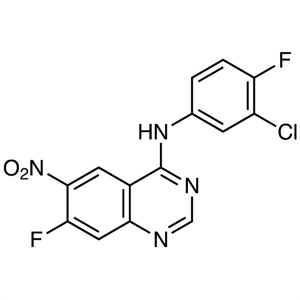
N-(3-Chloro-4-Fluorophenyl)-7-Fluoro-6-Nitroqui...
-
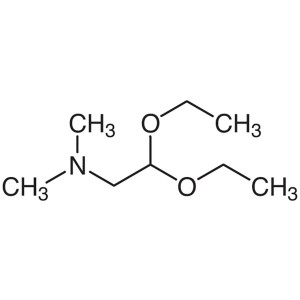
(Dimethylamino)acetaldehyde Diethyl Acetal CAS ...
-
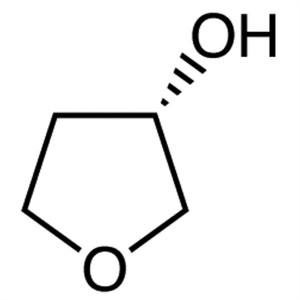
(S)-(+)-3-Hydroxytetrahydrofuran CAS 86087-23-2...
-
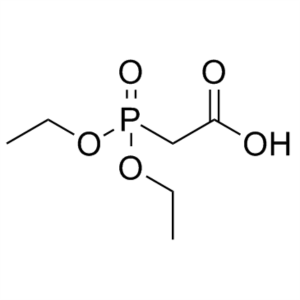
Diethylphosphonoacetic Acid CAS 3095-95-2 Purit...
-
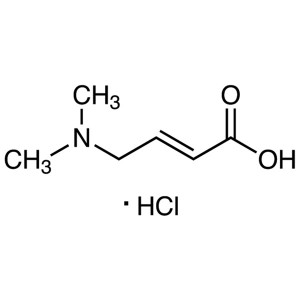
trans-4-Dimethylaminocrotonic Acid Hydrochlorid...
-
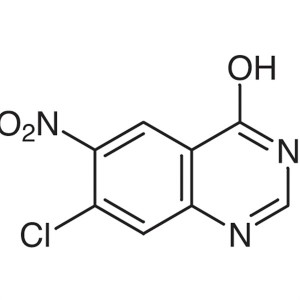
7-Chloro-6-Nitro-4-Hydroxyquinazoline CAS 53449...
-
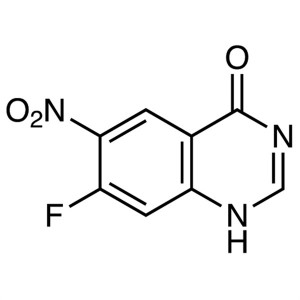
7-Fluoro-6-Nitroquinazolin-4(1H)-one CAS 162012...
-
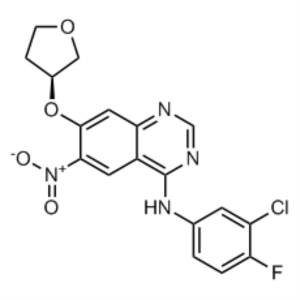
Afatinib Dimaleate Intermediate CAS 314771-88-5...
-
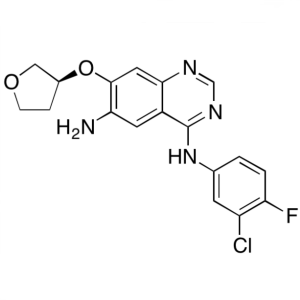
Afatinib Dimaleate Intermediate CAS 314771-76-1...
-
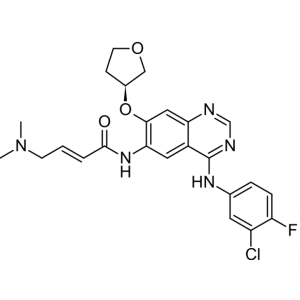
Afatinib CAS 439081-18-2 Purity >99.5% (HPLC) F...
-
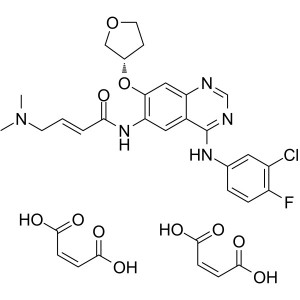
Afatinib Dimaleate CAS 850140-73-7 Purity >99.5...