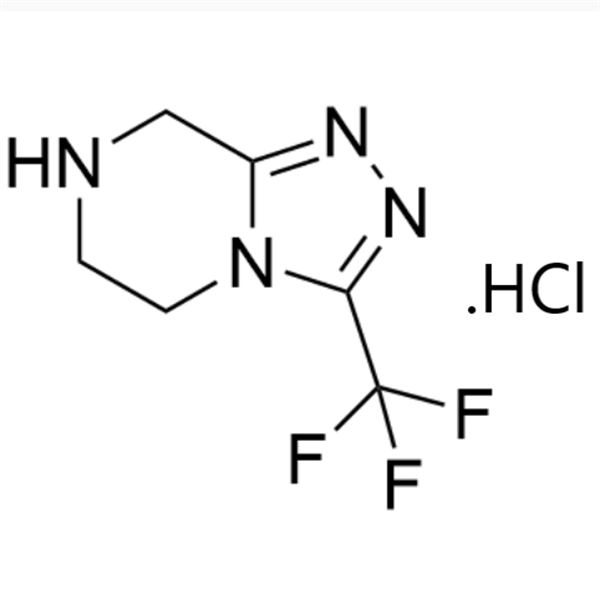
Sitagliptin Phosphate Monohydrate Intermediate CAS 486460-21-3 Purity >99.0% (HPLC) Factory High Quality
Supply Sitagliptin Phosphate Monohydrate Related Intermediates:
Sitagliptin API CAS 486460-32-6
Sitagliptin Phosphate Monohydrate API CAS 654671-77-9
2,4,5-Trifluorophenylacetic Acid CAS 209995-38-0
Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Butyric Acid CAS 486460-00-8
Sitagliptin Triazole Hydrochloride CAS 762240-92-6
Sitagliptin Phosphate Monohydrate Intermediate CAS 486460-21-3
| Chemical Name | 3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride |
| Synonyms | Sitagliptin Phosphate Monohydrate Intermediate |
| CAS Number | 486460-21-3 |
| CAT Number | RF-PI1194 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H7F3N4 |
| Molecular Weight | 192.14 |
| Boiling Point | 266℃ |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Light Yellow Powder |
| Identification | The UV absorption spectrum of the standard sample exhibits maximum&minimum at same wavelengths |
| Identification | HPLC: Retention time Similar |
| Loss on Drying | <2.00% |
| Ordinary Impurities | <1.00% |
| Purity / Analysis Method | >99.0% (HPLC) |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride (CAS: 486460-21-3) is an intermediate of Sitagliptin Phosphate Monohydrate (CAS: 654671-77-9) for the treatment of type II diabetes mellitus. Sitagliptin is approved by the FDA as an adjunct to diet and exercise to improve glycaemic control in patients with T2DM, either as a monotherapy, or in combination with Metformin or a peroxisome proliferatoractivated receptor-γ agonist (for example, thiazolidinediones) when the single agent does not provide adequate glycaemic control.
-
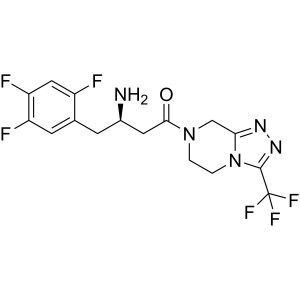
Sitagliptin CAS 486460-32-6 Purity >99.0% (HPLC...
-
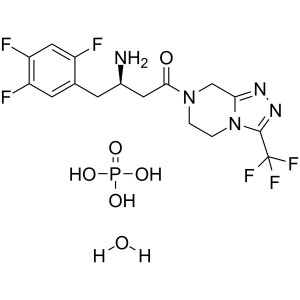
Sitagliptin Phosphate Monohydrate CAS 654671-77...
-
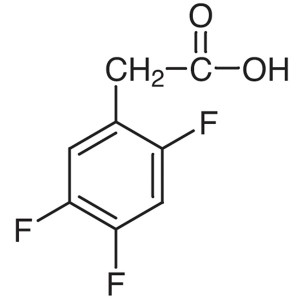
2,4,5-Trifluorophenylacetic Acid CAS 209995-38-...
-
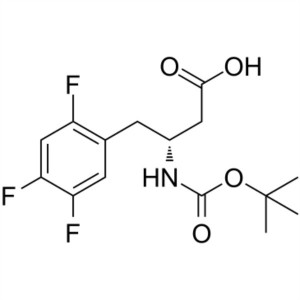
Boc-(R)-3-Amino-4-(2,4,5-Trifluoro-Phenyl)-Buty...
-
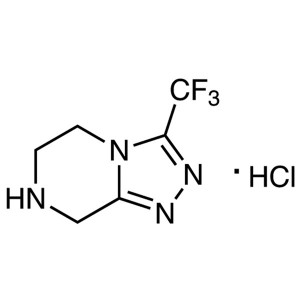
Sitagliptin Triazole Hydrochloride CAS 762240-9...
-
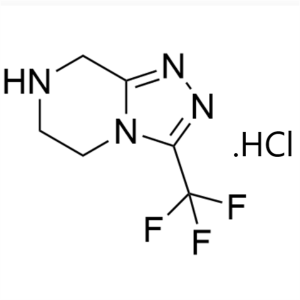
Sitagliptin Phosphate Monohydrate Intermediate ...
-
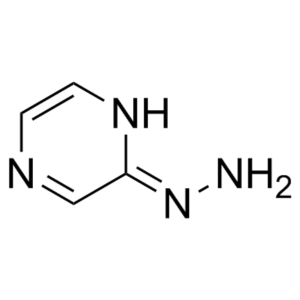
2-Hydrazinopyrazine CAS 54608-52-5 Purity >98.0...