Octreotide Acetate CAS 83150-76-9 Peptide Purity (HPLC) ≥98.0% API High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Octreotide Acetate (CAS: 83150-76-9) with high quality. Ruifu Chemical supplys a series of GMP peptides. Ruifu can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase Octreotide Acetate, Please contact: alvin@ruifuchem.com
| Chemical Name | Octreotide Acetate |
| Synonyms | SMS 201-995; Samilstin; Octreotide-LAR; |
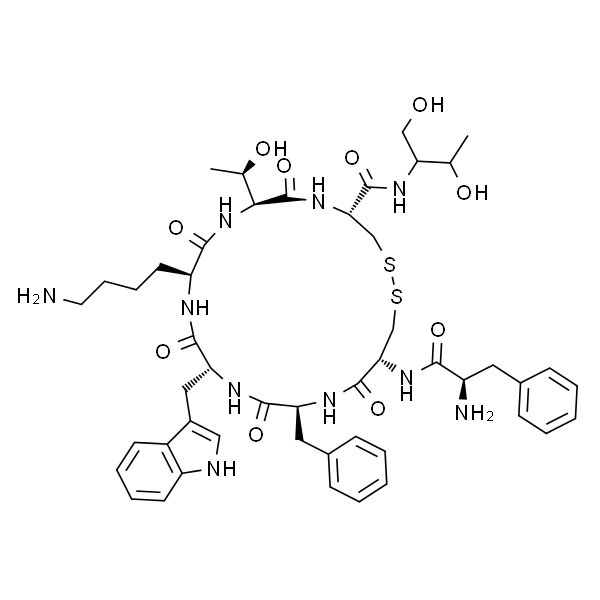
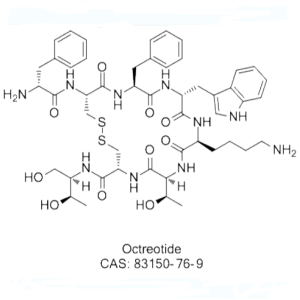
| Sequences | D-Phe-c[Cys-Phe-D-Trp-Lys-Thr-Cys]-Thr-ol |
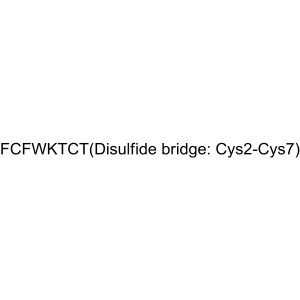
| SMILES | [FCFWKTCT(Disulfide Bridge: Cys2-Cys7)] |
| CAS Number | 83150-76-9 |
| Stock Status | In Stock, Production Scale Up to Kilograms |
| Molecular Formula | C49H66N10O10S2 |
| Molecular Weight | 1019.24 |
| Melting Point | >140℃(dec.) |
| Density | 1.39±0.10 g/cm3 |
| Solubility | Soluble in Acetic Acid, DMSO, and Methanol |
| Stability | Hygroscopic |
| Storage Temp. | Cool & Dry Place (2~8℃). Refrigerator |
| Document | COA, MS, HPLC, NMR, MSDS, etc. |
| Category | GMP Peptides |
| WGK Germany | 3 |
| Shipping Requirements | Ice Bags, Desiccant Bags |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White Powder | White Powder |
| Solubility | Soluble in Water or 1% Acetic Acid at a Concentration of ≥1mg/ml to Give a Clear Colorless Solution | Conforms |
| Amino Acid Analysis (By HPLC) | Thr: 0.7~1.1 | 0.9 |
| Cys: 1.0~2.2 | 1.9 | |
| Lys: 0.9~1.3 | 1.1 | |
| Phe: 1.8~2.2 | 1.9 | |
| Thr-OL: 0.6~1.3 | 0.9 | |
| Trp: 0.4~1.1 | 0.8 | |
| Peptide Purity (By HPLC) | ≥98.0% (by Area Integration) | 99.81% |
| Related Substances (By HPLC) | Total Impurities (%) ≤2.0% | 0.19% |
| Largest Single Impurity (%) ≤1.0% | 0.12% | |
| Acetate Content (HPLC) | 5.0~12.8% | 9.6% |
| Water Content (Karl Fischer) | ≤7.0% | 2.7% |
| TFA Content (Karl Fischer) | ≤0.25% | 0.13% |
| Specific Optical Rotation | -45.0°~-55.0° (C=0.5, 95% HAc) | -51.5° |
| Assay | 95.0~105.0% (Anhydrous, Acetic Acid Free) | 99.0% |
| Origin of Product | Synthetic | |
| Attention | Only for Research, Not for Human Use | |
| Conclusion | The product has been tested & complies with the specifications | |
| Usage | GMP Peptides; Active Pharmaceutical Ingredient (API) | |
Package: Plastic vial (dedicated for peptide packing) or glass vial, quantity according to customer's detail requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.

Octreotide Acetate (Sandostatin) is a synthetic peptide analogue of the hormone somatostatin, it is a long-acting somatostatin analog indicated for symptomatic control in acromegaly and gastroenteropancreatic tumors. Other potential uses under investigation include diabetes, psoriasis and Alzheimer's disease. Octreotide Acetate (Sandostatin) is a synthetic peptide analogue of the hormone somatostatin. Its actions include inhibition of the pituitary secretion of growth hormone and an inhibition of pancreatic islet cell secretion of insulin and glucagon. Unlike somatostatin, which has a plasma half-life of a few minutes, Octreotide has a plasma elimination half-life of 1 to 2 hours. Excretion of the drug is primarily renal. Octreotide reduces gastrointestinal motility and inhibits contraction of the gall bladder.
Peptide drugs Novartis Sandostatin (Octreotide Acetate), is a synthetic natural somatostatin octapeptide derivatives, it retains a pharmacological effect similar to that of somatostatin, and the effect is long-lasting. Indications for Octreotide Acetate include acromegaly; Alleviation of symptoms and signs associated with functional gastroenteropancreatic endocrine neoplasia; And efficacy in Carcinoid Tumors presenting with carcinoid syndrome, VIP tumors, and glucagon tumors. In addition, Octreotide Acetate for gastrinomas/Zollinger-Ellison syndrome (usually in combination with proton pump inhibitors or H2 receptor blockers), Insulinomas (preoperative prevention of hypoglycemia and maintenance of normal blood sugar), the effective rate of growth hormone releasing factor tumor is about 50%.
Octreotide Acetate is a national health insurance Class B drug, which is a synthetic natural octapeptide derivative of somatostatin, compared with natural somatostatin, the half-life of somatostatin is obviously prolonged and has the same pharmacological effects, including inhibition of growth hormone function, inhibition of gastric acid, pancreatic enzyme, glucagon and insulin secretion, and reduction of visceral blood flow, decreased gastrointestinal motility. Compared with native somatostatin, octreotide has a long half-life, is more selective for inhibition of growth hormone secretion than for inhibition of insulin secretion, and does not cause rebound hypersecretion of the hormone. 1, Control surgery or radiation therapy can not adequately control the disease in patients with acromegaly symptoms and reduce the patient's growth hormone (GH) insulin-like growth factor -1(1GF-1) plasma levels. Sandostatin may also be used to treat patients with acromegaly who are unable or unwilling to undergo surgery, or to treat patients with acromegaly who have not yet had radiation therapy. 2. Alleviation of symptoms associated with functional gastroenteropancreatic (GEP) endocrine tumors such as carcinoid tumors with carcinoid syndrome manifestations. Sandostatin is not an anti-cancer drug and does not cure these patients. 3, Prevention of complications after pancreatic surgery. 4, Liver cirrhosis patients with gastric-esophageal variceal bleeding caused by emergency treatment, hemostasis and prevention of rebleeding, Sandostatin should be combined with endoscopic sclerotherapy and other special treatment.
The side effects of this product are Abdominal Pain, abdominal distension, Diarrhea, steatorrhea; Gallstone formation; Glucose tolerance may decrease or worsen in the early stage of treatment, and then can be improved; Long-term application without endogenous antibody production, there was no obvious rebound or adverse reaction when the drug was stopped or rapidly reduced. 2~8 deg C for long-term storage.
-
Deslorelin Acetate CAS 57773-65-6 GnRH Agonist ...
-
Cetrorelix Acetate CAS 130143-01-0 GnRH Antagon...
-
Octreotide Acetate CAS 83150-76-9 Peptide Purit...
-
Melanotan II (MT-II) CAS 121062-08-6 Peptide Pu...
-
Desmopressin Acetate CAS 16789-98-3 Peptide Pur...
-
Histrelin Acetate CAS 76712-82-8 Peptide Purity...