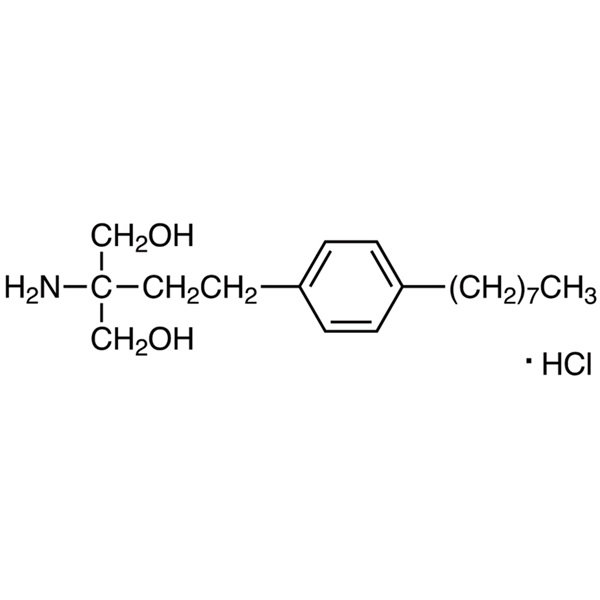
Fingolimod Hydrochloride CAS 162359-56-0 Purity ≥99.0% (HPLC) API Factory High Purity
Manufacturer with High Purity and Stable Quality
Chemical Name: Fingolimod Hydrochloride
CAS: 162359-56-0
Fingolimod Hydrochloride is a FDA approved drug for Multiple Sclerosis treatment.
API High Quality, Commercial Production
| Chemical Name | Fingolimod Hydrochloride |
| Synonyms | Fingolimod HCl; FTY 720 |
| CAS Number | 162359-56-0 |
| CAT Number | RF-API39 |
| Stock Status | In Stock, Production Scale Up to Hundreds of Kilograms |
| Molecular Formula | C19H34ClNO2 |
| Molecular Weight | 343.93 |
| Melting Point | 102.0~107.0°C |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Identification | HPLC |
| Melting Point | 102.0~106.0℃ |
| Solubility | Completely Soluble in Water |
| Purity / Analysis Method | ≥99.0% (HPLC) |
| Moisture (K.F) | ≤0.50% |
| Residue on Ignition | ≤0.20% |
| Heavy Metals | ≤20ppm |
| Residual Solvents | |
| Dichloromethane | ≤0.05% |
| Ethyl Acetate | ≤0.50% |
| Ethanol | ≤0.50% |
| Methanol | ≤0.30% |
| Toluene | ≤0.089% |
| THF | ≤0.50% |
| DMF | ≤0.088% |
| Relative Substances | |
| Single Impurity | ≤0.50% |
| Total Impurities | ≤1.0% |
| Test Standard | Enterprise Standard |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Fingolimod Hydrochloride (CAS: 162359-56-0) with high quality.
Fingolimod Hydrochloride is the first oral drug for the treatment of multiple sclerosis. It is successfully developed by the pharmaceutical company Novartis, and it has been approved for marketing by the US Food and Drug Administration (FDA). Multiple sclerosis is a debilitating neurological disease that can cause the patient to lose a sense of balance, appear muscle spasms and other movement disorders. Fingolimod Hydrochloride is a sphingosine-l-phosphate (S1PR) receptor modulator. After phosphorylation, It is bound to s1P receptor that is on the surface of lymphocyte, which will change lymphocyte migration, and promote cells into the lymphatic tissue, and prevent lymphocytes from leaving the lymphoid tissue and get into the graft. Thereby, it will prevent these cells from infiltrating the central nervous system (CNS), which achieves the effect of immunosuppression.
-
Fingolimod Hydrochloride CAS 162359-56-0 Purity...
-
Cisatracurium Besylate CAS 96946-42-8 Assay 95....
-
Citicoline Sodium Salt Hydrate CAS 33818-15-4 A...
-
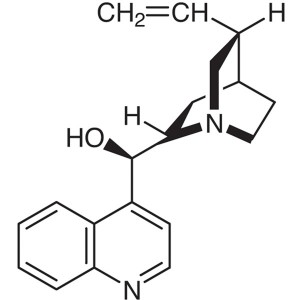
Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...
-
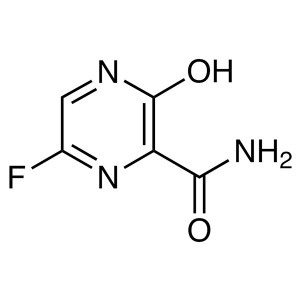
Favipiravir CAS 259793-96-9 T-705 Purity ≥99.0%...
-
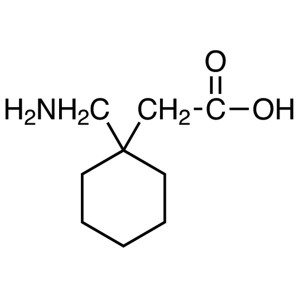
Gabapentin CAS 60142-96-3 Purity >99.5% (HPLC) ...
-
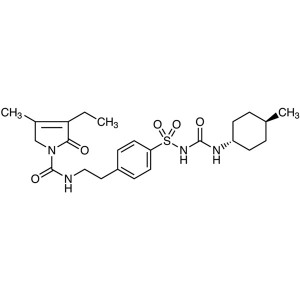
Glimepiride CAS 93479-97-1 Assay 98.0%~102.0% A...
-
Imatinib Mesylate CAS 220127-57-1 Assay 98.0%~1...
-
Ibrutinib CAS 936563-96-1 Purity >99.5% (HPLC) API
-
Lapatinib Base CAS 231277-92-2 Purity ≥99.0% (H...
-
Olaparib AZD-2281 CAS 763113-22-0 Purity ≥99.0%...
-
Perindopril Erbumine CAS 107133-36-8 Purity >99...
-
Caspofungin Acetate Cancidas CAS 179463-17-3 AP...
-
Cefotaxime Sodium Salt CAS 64485-93-4 Assay ≥91...
-
Cholestyramine CAS 11041-12-6 USP API Factory H...
-
CAS 842133-18-0 Purity ≥99.0% (HPLC) Type 2 Dia...