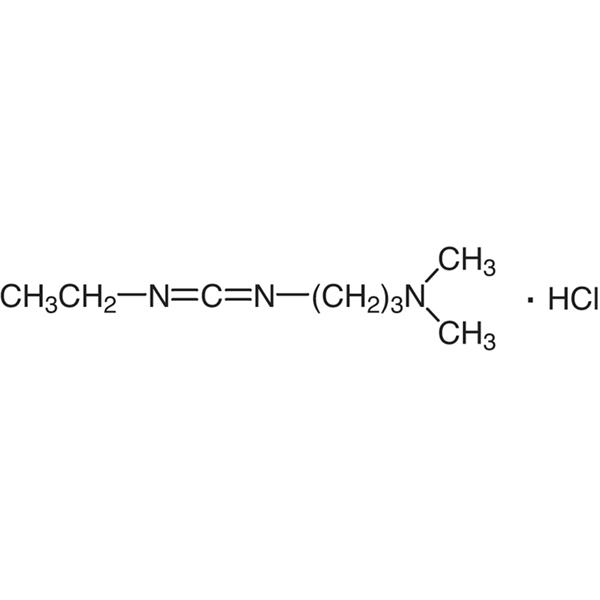
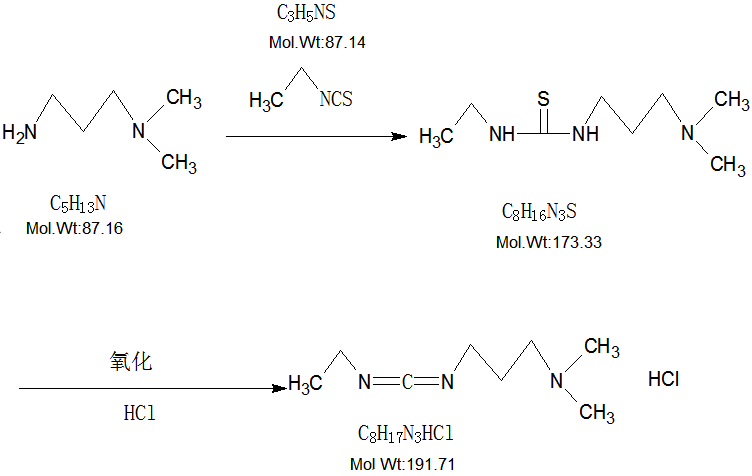
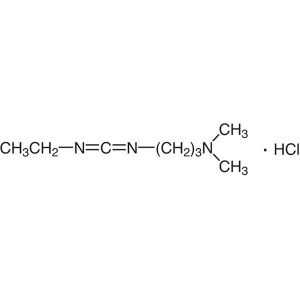
EDC·HCl CAS 25952-53-8 Coupling Reagent Purity >99.0% (T) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride (EDC·HCl) (CAS: 25952-53-8) with high quality. Ruifu Chemical supplys a series of protecting reagents and coupling reagents. Ruifu can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase EDC·HCl, Please contact: alvin@ruifuchem.com
| Chemical Name | 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride |
| Synonyms | EDC·HCl; EDAC·HCl; EDCI·HCl; EDC Hydrochloride; EDAC Hydrochloride; N-(3-Dimethylaminopropyl)-N′-Ethylcarbodiimide Hydrochloride; 1-Ethyl-3-(3-Dimethylaminopropyl)carbodiimide Hydrochloride; N-Ethyl-N'-(3-dimethylaminopropyl)carbodiimide Hydrochloride; Water Soluble Carbodiimide |
| Stock Status | In Stock, Production Capacity 500 Metric Tons per Year |
| CAS Number | 25952-53-8 |
| EDC CAS Number | 1892-57-5 |
| Molecular Formula | C8H17N3·HCl |
| Molecular Weight | 191.70 g/mol |
| Melting Point | 110.0~115.0℃ |
| Density | 0.877 g/mL at 20℃(lit.) |
| Refractive Index n20/D | 1.461 |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Hygroscopic. Moisture Sensitive, Heat Sensitive |
| Water Solubility | Completely Soluble in Water |
| Solubility | Soluble in Dimethylformamide, Alcohol |
| Storage Temp. | Cool & Dry Place (<5℃) |
| COA & MSDS | Available |
| Category | Coupling Reagents |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White Crystalline Powder | Complies |
| Melting Point | 110.0~115.0℃ | 112.0~114.0℃ |
| Water by Karl Fischer | <0.50% | 0.10% |
| Residue on Ignition | <0.10% | 0.04% |
| Purity (Argentmetric Titration) | >99.0% | 99.70% |
| Purity / Analysis Method | >99.0% (HPLC) | Complies |
| Infrared Spectrum | Conforms to Structure | Complies |
| Solubility in H2O (100 mg/mL) | Colorless to Very Faint Yellow, Clear | Pass |
| Conclusion | The product has been tested & complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Moisture Sensitive. Store in sealed containers at cool and dry (<5℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
Properties: This product is white crystalline powder.
2. Content determination:
2.1 Instruments and reagents: potentiometric titrator, nitric acid solution (12.5 wt%), AgNO3 standard solution (0.1mol/L)
2.2 Sample preparation and operation:
200 to 220mg was dissolved in 40ml of distilled water, and after 5ml of nitric acid solution was added, the solution was standard dissolved with silver nitrate
The fluid was titrated to the end point. The formula for calculating product content is as follows:
X = (VAgNO3*19.171)/W,
X- The percentage of EDC.HCl, 100%.
V-The volume consumed by the AgNO3 standard solution, ml
19.171-1ml AgNO3 standard solution (0.1mol/L) consumed EDC.HCl mass, mg;
W - sample mass, mg;
The method was performed three times in parallel and the average was taken, and the error must not exceed 0.3%. Product content should be >99.0%.
3. Melting point: according to the melting point determination method (Appendix VII C of Chinese Pharmacopoeia 2010 edition), the melting point of this product should be 110.0~115.0℃.
4. Water content determination: according to the moisture measurement method (KF method) to determine, the water content of this product should be <0.50%.
5. Storage: Store in a dark and cool dry place (<5℃).
6. Review period: one year
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R34 - Causes burns
R36/37/38 - Irritating to eyes, respiratory system and skin.
R41 - Risk of serious damage to eyes
R37/38 - Irritating to respiratory system and skin.
R20/21/22 - Harmful by inhalation, in contact with skin and if swallowed.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S37/39 - Wear suitable gloves and eye/face protection
S36 - Wear suitable protective clothing.
UN IDs UN 2735 8/PG 3
WGK Germany 3
RTECS FF2200000
FLUKA BRAND F CODES 1-3-10
TSCA Y
HS Code 2925290090
Hazard Note Irritant
Toxicity LD50 intravenous in mouse: 56mg/kg
1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride (EDC·HCl) (CAS: 25952-53-8) is a white crystalline powder, easily deliquescent, soluble in water, soluble in ethanol, used as an activating reagent for carboxyl groups in amide synthesis, also used for activating phosphate groups, cross-linking of proteins and nucleic acids, and the production of immunocouplers. EDC-HCl is used in the pH range of 4.0-6.0 and is often used in conjunction with N-Hydroxysuccinimide (NHS) or N-Hydroxysulfosuccinimide sodium salt to improve coupling efficiency.
EDC·HCl is water-soluble carbodiimide, widely used for peptide coupling. EDC·HCl is used as a carboxyl activating agent. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis. The major advantage of EDAC coupling is the easy removal of excess reagent and the corresponding urea by washing with dilute acid or water. Carbodiimides catalyze the formation of amide bonds, carboxylic acids, and amines by activating the carboxylate to form an O-acylurea. This intermediate can be attacked by an amine directly to form an amide. EDAC is released as a soluble urea derivative.
EDC·HCl can be used for the synthesis of amides. It is used as a coupling agent in the synthesis of esters from carboxylic acids using dimethylaminopyridine as the catalyst.
There are mainly three commonly used condensing agents: DCC, DIC and EDC·HCl. The use of this type of condensing agent generally requires the addition of acylation catalysts or activators, such as DMAP, HOBt, etc. Since the addition intermediate of the acid to the carbodiimide in the first stage of the reaction is not stable, if the acylation catalyst is not used to convert it into the corresponding active ester or active amide, it will be rearranged into the corresponding stable by-product of urea (Path B). Condensation activator: There are the following types of commonly used condensation activator. At present, 4-N,N-dimethylpyridine (DMAP) has been widely used to catalyze various acylation reactions. Sometimes 4-PPY can be used when the catalytic effect of DMAP is not good. According to relevant literature, its catalytic ability is about a thousand times higher than DMAP. among the three commonly used condensers, DCC and DIC are cheaper. Generally DCC and DMAP are used together. One of the biggest disadvantages of using DCC is that the other product of the reaction, dicyclohexylurea, has a small solubility in the general organic phase but is slightly soluble, so it is difficult to remove it thoroughly through some commonly used purification methods, recrystallization, column chromatography, etc.; because the solubility of dicyclohexylurea in ether is relatively small than other solvents, therefore, the treatment of this type of reaction generally evaporates the reaction solvent and adds ether, and filters out most of the dicyclohexylurea before further treatment. Because the diisopropylurea produced by DIC has better solubility in general organic solvents, it is generally used in combinatorial chemistry solid-phase synthesis. EDCI is currently used most in medicinal chemistry. One of its main characteristics is that the urea generated after the reaction is water-soluble and easy to be washed off. Generally, EDCI is combined with HOBt (note: this reaction HOBt is generally indispensable, otherwise it may lead to too low condensation yield).
-
EDC·HCl CAS 25952-53-8 Coupling Reagent Purity ...
-
BOP Reagent CAS 56602-33-6 Peptide Coupling Rea...
-
BOP-Cl CAS 68641-49-6 Peptide Coupling Reagent ...
-
CDI CAS 530-62-1 N,N’-Carbonyldiimidazole...
-
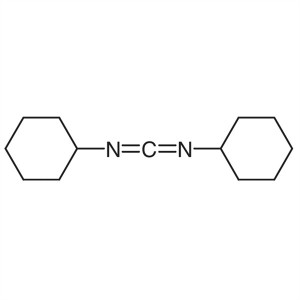
DCC CAS 538-75-0 N,N’-Dicyclohexylcarbodi...
-
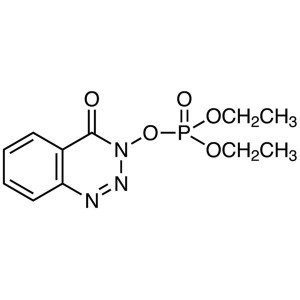
DEPBT CAS 165534-43-0 Peptide Coupling Reagent ...
-
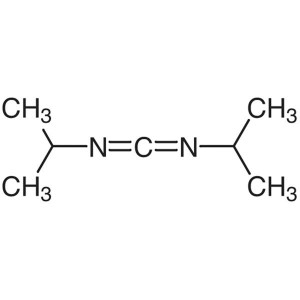
DIC CAS 693-13-0 N,N’-Diisopropylcarbodii...
-
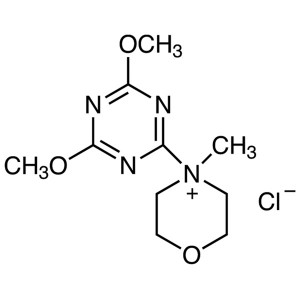
DMTMM CAS 3945-69-5 Coupling Reagent Purity >99...
-
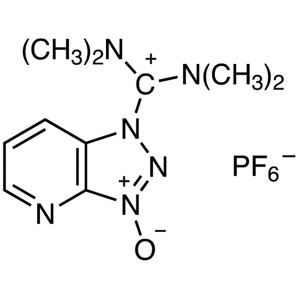
HATU CAS 148893-10-1 Peptide Coupling Reagent P...
-
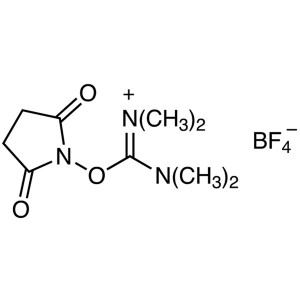
TSTU CAS 105832-38-0 Purity >99.0% (HPLC) Facto...
-
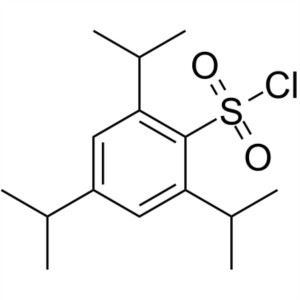
TPSCl CAS 6553-96-4 2,4,6-Triisopropylbenzenesu...
-
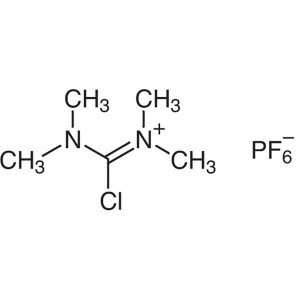
TCFH CAS 94790-35-9 Purity >99.0% (HPLC) Factor...
-
Bis(4-Nitrophenyl) Carbonate (NPC) CAS 5070-13-...
-
PyAOP CAS 156311-83-0 Purity >99.0% (HPLC) Coup...
-
PyBrOP CAS 132705-51-2 Purity >99.0% (HPLC) Fac...
-
HOAt CAS 39968-33-7 1-Hydroxy-7-Azabenzotriazol...