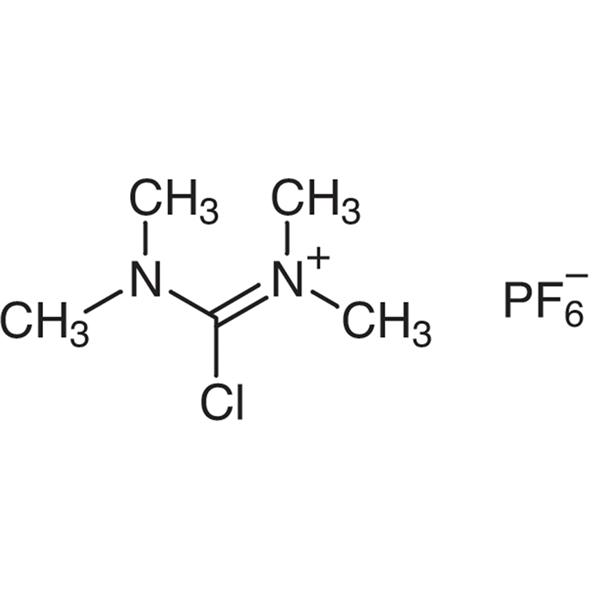
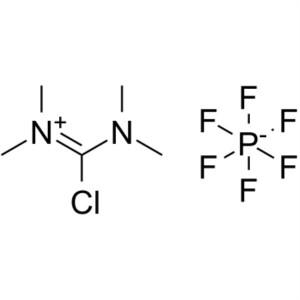
TCFH CAS 94790-35-9 Purity >99.0% (HPLC) Factory Coupling Reagents
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of TCFH (CAS: 94790-35-9) with high quality. Ruifu Chemical supplys a series of protecting reagents and coupling reagents. Ruifu can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase TCFH, Please contact: alvin@ruifuchem.com
| Chemical Name | N,N,N',N'-Tetramethylchloroformamidinium Hexafluorophosphate |
| Synonyms | TCFH; Chloro-N,N,N',N'-Tetramethylformamidinium Hexafluorophosphate; N-[Chloro(dimethylamino)methylene]-N-Methyl-Methanaminium Hexafluorophosphate; Tetramethylchloroformamidinium Hexafluorophosphate |
| Stock Status | In Stock, Mass Production |
| CAS Number | 94790-35-9 |
| Molecular Formula | C5H12ClF6N2P |
| Molecular Weight | 280.58 g/mol |
| Melting Point | 99.0~108.0℃ |
| Density | 1.58 |
| Sensitive | Moisture Sensitive, Heat Sensitive |
| Storage Temp. | Cool & Dry Place (2~8℃) |
| COA & MSDS | Available |
| Category | Coupling Reagents |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Crystalline Powder | Complies |
| Melting Point | 99.0~108.0℃ | 102.4~103.6℃ |
| Loss on Drying | <0.50% | 0.15% |
| Purity / Analysis Method | >99.0% (HPLC) | 99.9% |
| Infrared Spectrum | Conforms to Structure | Complies |
| Proton NMR Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry (2~8℃) warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R36/37/38 - Irritating to eyes, respiratory system and skin.
R40 - Limited evidence of a carcinogenic effect
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
S22 - Do not breathe dust.
WGK Germany 3
FLUKA BRAND F CODES 3-10-21
N,N,N',N'-Tetramethylchloroformamidinium Hexafluorophosphate (TCFH) (CAS: 94790-35-9), coupling reagent for peptide synthesis and starting material for preparing other coupling reagents. TCFH is a good non-hygroscopic peptide synthesis coupling reagent. As a general activation reagent of solid phase chemistry, TCFH mainly promotes the formation of carboxylic acid amine derivatives rather than amide in the coupling process of amino acids. It is very useful for the coupling of very hindered amino acids, and has the characteristics of simple and convenient synthesis process and long shelf life.
TCFH is a safe, affordable reagent for ester and difficult amide synthesis.
TCFH as a reagent has several advantages beside being economical:
– Due to is high coupling potency, it might be used in reactions previously only accomplished using carbodiimide-mediated couplings, extending in this manner its application for the formation of esters, diacyl amides and acylation of non-reactive amines
– It is solid, reasonably stable compound, when stored around 0°C
– Using the widely available coupling additives the chemist can find optimal reagent ratio to balance between the product yield and the side reactions including epimerization.
Over a period of 2 minutes, to 1,1,3,3-Tetramethylurea (2.5 mL, 21 mmol)/10 mL toluene solution a small portion of oxalyl chloride (2.0 mL, 23 mmol) was added while the reaction mixture was stirred at ice-water bath temperature. After stirring at room temperature for 18 hours, the reaction mixture was concentrated under reduced pressure to a volume of about 5 mL and the resulting precipitate was collected by filtration. The collected solid was dissolved in about 20 mL of water and then treated with potassium hexafluorophosphate (3.8g, 21 mmol)/50 mL of aqueous solution while the mixture was vigorously stirred at room temperature, to obtain a white precipitate. The precipitate was collected by filtration and dried under vacuum. The solid was then treated with about 8 mL of acetone and filtered to remove insoluble material. The resulting filtrate was added in small portions to 60 mL of diethyl ether with vigorous stirring at room temperature. The desired product was collected by filtration and dried under vacuum to give 4.5g (76%).
-
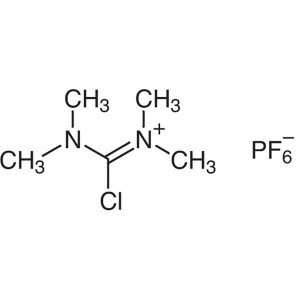
TCFH CAS 94790-35-9 Purity >99.0% (HPLC) Factor...
-
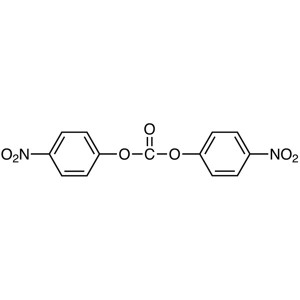
Bis(4-Nitrophenyl) Carbonate (NPC) CAS 5070-13-...
-
TPSCl CAS 6553-96-4 2,4,6-Triisopropylbenzenesu...
-
TSTU CAS 105832-38-0 Purity >99.0% (HPLC) Facto...
-
PyAOP CAS 156311-83-0 Purity >99.0% (HPLC) Coup...
-
PyBrOP CAS 132705-51-2 Purity >99.0% (HPLC) Fac...
-
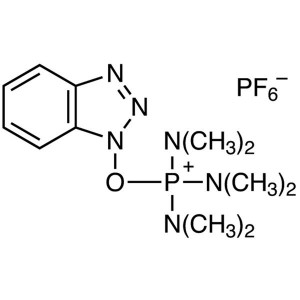
BOP Reagent CAS 56602-33-6 Peptide Coupling Rea...
-
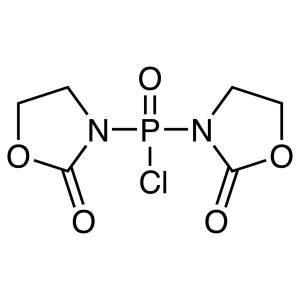
BOP-Cl CAS 68641-49-6 Peptide Coupling Reagent ...
-
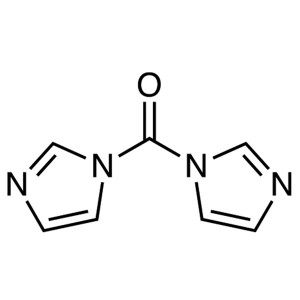
CDI CAS 530-62-1 N,N’-Carbonyldiimidazole...
-
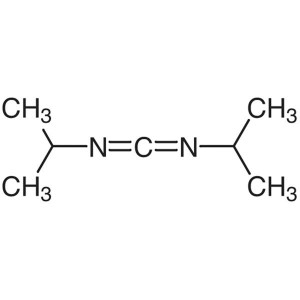
DIC CAS 693-13-0 N,N’-Diisopropylcarbodii...
-
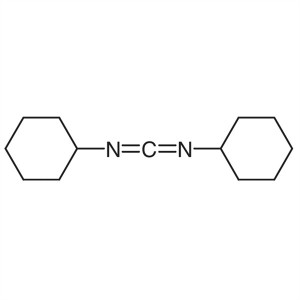
DCC CAS 538-75-0 N,N’-Dicyclohexylcarbodi...
-
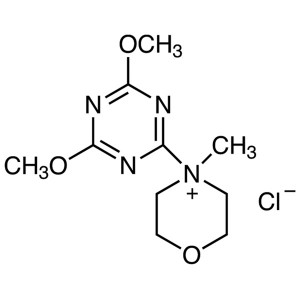
DMTMM CAS 3945-69-5 Coupling Reagent Purity >99...
-
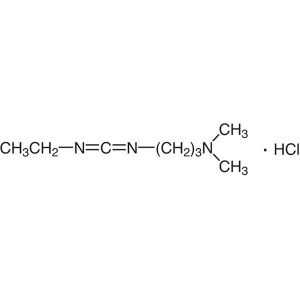
EDC·HCl CAS 25952-53-8 Coupling Reagent Purity ...
-
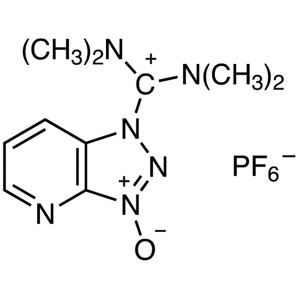
HATU CAS 148893-10-1 Peptide Coupling Reagent P...
-
HBTU CAS 94790-37-1 Peptide Coupling Reagent Pu...
-
HCTU CAS 330645-87-9 Peptide Coupling Reagent P...