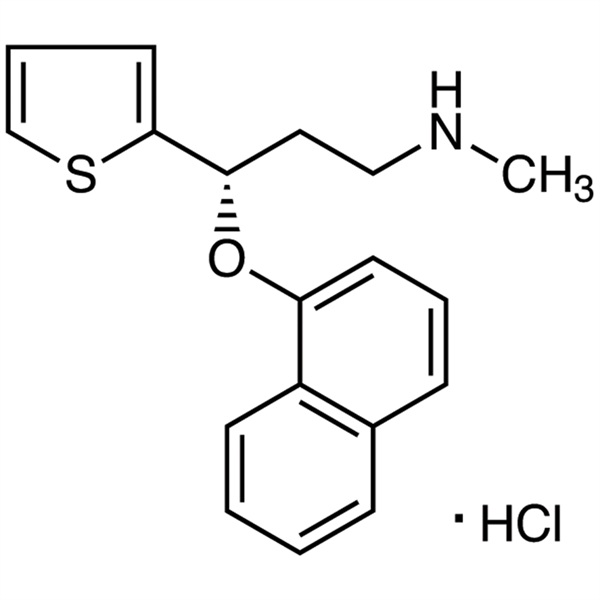
Duloxetine Hydrochloride CAS 136434-34-9 Purity >99.0% (HPLC) Anti-Depressant
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Duloxetine Hydrochloride (CAS: 136434-34-9) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Duloxetine Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Duloxetine Hydrochloride |
| Synonyms | Duloxetine HCl; (S)-Duloxetine HCl; LY-248686 Hydrochloride; (RS)-Duloxetine Hydrochloride; (S)-(+)-N-Methyl-3-(1-Naphthyloxy)-3-(2-Thienyl)propylamine Hydrochloride; (S)-N-Methyl-3-(Naphthalen-1-yloxy)-3-(Thiophen-2-yl)propan-1-Amine HCl; (γS)-N-Methyl-γ-(1-Naphthalenyloxy)-2-Thiophenepropanamine Hydrochloride; (+)-(S)-N-Methyl-γ-(1-Naphthyloxy)-2-Thiophenepropylamine Hydrochloride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 136434-34-9 |
| Related CAS | 116539-59-4 (Free Base) |
| Molecular Formula | C18H19NOS·HCl |
| Molecular Weight | 333.88 g/mol |
| Melting Point | 166.0~174.0℃ |
| Sensitive | Air Sensitive, Light Sensitive |
| Solubility | Soluble in Methanol |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Off-White Crystalline Powder | White Crystalline Powder |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Chloride Test | Meets the Requirements | Conforms |
| Specific Rotation [α]D25 | +119.0°~+129.0° (C=1 in MeOH) | +121.6° |
| Melting Range | 166.0~174.0℃ | 168.0~170.0℃ |
| Loss on Drying | ≤0.50% (105℃ for 3 h) | 0.23% |
| Residue on Ignition | ≤0.20% | 0.05% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Isomer | ≤0.50% | 0.28% |
| Duloxetine Related Compound F | ≤0.50% | <0.50% |
| Any Individual Unspecified Impurity | ≤0.10% | <0.10% |
| Total impurities | ≤0.60% | <0.60% |
| Residual Solvents | ||
| Ethyl Acetate | ≤0.50% | Not Detected |
| Dichloromethane | ≤0.06% | Not Detected |
| Ethanol | ≤0.50% | 0.10% |
| Purity / Analysis Method | >99.0% (HPLC) | 99.5% |
| Conclusion | Tested and complies with the given specifications | |
| Application | Anti-Depressant | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Duloxetine Hydrochloride
C18H19NOS · HCl 333.88
2-Thiophenepropanamine, N-methyl-γ-(1-naphthalenyloxy)-, hydrochloride, (S)-;
(+)-(S)-N-Methyl-γ-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride [136434-34-9].
DEFINITION
Duloxetine Hydrochloride contains NLT 97.0% and NMT 102.0% of C18H19NOS·HCl, calculated on the dried basis.
IDENTIFICATION
• A. INFRARED ABSORPTION <197K>
Sample solution: 5 mg/mL in methanol
Acceptance criteria: Meets the requirements
• B. The retention time of the major peak in the sample solution corresponds to that of the Duloxetine S-isomer from the System suitability solution in the test for Limit of Duloxetine Related Compound A.
• C. IDENTIFICATION TESTS-GENERAL, Chloride <191>: Meets the requirements
ASSAY
• PROCEDURE
Protect solutions of duloxetine from light.
Buffer: 2.9 g/L of phosphoric acid in water. Adjust with sodium hydroxide solution to a pH of 2.5. To each L of this solution add 10.3 g of sodium 1-hexanesulfonate monohydrate, and dissolve.
Mobile phase: Acetonitrile, n-propanol, and Buffer (13:17:70)
Diluent: Acetonitrile and water (25:75)
System suitability solution: 0.2 mg/mL of USP Duloxetine Hydrochloride RS in Mobile phase. Heat the solution to at least 40° for a minimum of 1 h. [NOTE-The resulting solution contains duloxetine impurity B, duloxetine impurity C, duloxetine impurity D, duloxetine impurity E, and duloxetine related compound F.]
Standard solution: 0.1 mg/mL of USP Duloxetine Hydrochloride RS in Diluent
Sample solution: 0.1 mg/mL of Duloxetine Hydrochloride in Diluent
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L7
Column temperature: 40 ± 3°
Flow rate: 1 mL/min
Injection size: 10 µL
Run time: 2 times the retention time of Duloxetine
System suitability
Sample: System suitability solution
[NOTE-See Table 1 for relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between Duloxetine and Duloxetine related compound F peaks
Tailing factor: NMT 1.5 for the duloxetine peak
Relative standard deviation: NMT 1.0% for the Duloxetine peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of duloxetine hydrochloride (C18H19NOS·HCl) in the portion of sample taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Duloxetine Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Duloxetine Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%~102.0% on the dried basis
IMPURITIES
• HEAVY METALS, Method II <231>: NMT 10 ppm
• RESIDUE ON IGNITION <281>: NMT 0.2%
• ORGANIC IMPURITIES
Protect solutions of Duloxetine from light.
Buffer, Mobile phase, Diluent, and System suitability solution: Proceed as directed in the Assay.
Sensitivity solution: 0.2 µg/mL of USP Duloxetine Hydrochloride RS in Diluent
Sample solution: 0.2 mg/mL of Duloxetine Hydrochloride in Diluent
Chromatographic system: Proceed as directed in the Assay
Run time: 2.4 times the retention time of Duloxetine
System suitability
Samples: System suitability solution and Sensitivity solution
[NOTE-See Table 1 for relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between duloxetine impurity C and duloxetine impurity D; NLT 1.5 between Duloxetine and Duloxetine related compound F, System suitability solution
Tailing factor: NMT 1.5 for the duloxetine peak, System suitability solution
Relative standard deviation: NMT 1.0% for the Duloxetine peak, System suitability solution
Signal-to-noise ratio: NLT 20 for the Duloxetine peak, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of any individual impurity in the portion of Duloxetine Hydrochloride taken:
Result = (rU/rT) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of the responses of all the peaks from the Sample solution
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1.
Table 1
Name Relative Retention Tme Relative Response Factor Acceptance Criteria NMT (%)
Duloxetine impurity Ba,g 0.15 0.36 -
Duloxetine impurity Cb,g 0.43 1.0 -
Duloxetine impurity Dc,g 0.48 1.8 -
Duloxetine impurity Ed,g 0.74 1.0 -
Duloxetine 1.0 - -
Duloxetine related compound Fe 1.1 1.0 0.5
Duloxetine impurity Gf,g 1.4 0.51 -
Any individual unspecified impurity - 1.0 0.1
Total impurities - - 0.6
a 3-(Methylamino)-1-(thiophen-2-yl)propan-1-ol
b 4-[3-(Methylamino)-1-(thiophen-2-yl)propyl]naphthalen-1-ol.
c Naphthalen-1-ol.
d 1-(3-(Methylamino)-1-(thiophen-2-yl)propyl)naphthalen-2-ol.
e (S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-3-yl)propan-1-amine
f 1-Fluoronaphthalene
g Controlled at Any individual unspecified impurity level.
• LIMIT OF DULOXETINE RELATED COMPOUND A
Mobile phase: Hexane and isopropyl alcohol (83:17). To 1 L of this mixture add 2 mL of diethylamine.
System suitability solution: 0.1 mg/mL each of USP Duloxetine Hydrochloride RS and USP Duloxetine Related Compound A RS in Mobile phase. Sonication may be used to aid in dissolution.
Sensitivity solution: 0.1 µg/mL of USP Duloxetine Hydrochloride RS in Mobile phase
Sample solution: 0.1 mg/mL of Duloxetin Hydrochloride in Mobile phase. Sonication may be used to aid in dissolution.
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 230 nm
Column: 4.6-mm × 25-cm; 5-µm packing L40
Column temperature: 40°
Flow rate: 1 mL/min
Injection size: 10 µL
Run time: 2 times the retention time of Duloxetine
System suitability
Samples: Sensitivity solution and System suitability solution
[NOTE-The relative retention times for Duloxetine and Duloxetine related compound A are 1.0 and 1.3, respectively.]
Suitability requirements
Resolution: NLT 3.5 between Duloxetine and Duloxetine related compound A, System suitability solution
Tailing: Between 0.8 and 1.5 each for Duloxetine and Duloxetine related compound A peaks, System suitability solution
Relative standard deviation: NMT 5.0% for the Duloxetine peak, System suitability solution
Signal-to-noise ratio: NLT 3, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of Duloxetine related compound A in the portion of Duloxetine Hydrochloride taken:
Result = (rU/rT) × 100
rU = peak response for Duloxetine related compound A from the Sample solution
rT = sum of the responses of Duloxetine and Duloxetine related compound A peaks from the Sample solution
Acceptance criteria: NMT 0.5%
SPECIFIC TESTS
• LOSS ON DRYING <731>: Dry at 105° for 3 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Protect from light. Store at room temperature.
• USP REFERENCE STANDARDS <11>:
USP Duloxetine Hydrochloride RS
USP Duloxetine Related Compound A RS
(R)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-amine hydrochloride.
C18H19NOS·HCl 333.88 2S (USP35)
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Codes Xi,Xn,T,F
Risk Statements 36/37/38-22-39/23/24/25-23/24/25-11
Safety Statements 26-36/37-45-16-7
RIDADR UN1230 - class 3 - PG 2 - Methanol, solution
WGK Germany 3
RTECS XN0258000
HS Code 2934999099
Duloxetine Hydrochloride (CAS: 136434-34-9) is the salt form of antidepressant drug Duloxetine. It was successfully developed by Eli Lilly Company. Its pharmacological effect is the same as Duloxetine. Duloxetine Hydrochloride is a new kind of selective dual inhibitor of 5 hydroxy trptamine (5-HT) and norepinephrine reuptake, and thus having antidepressant effect, while also having inhibitory effect on the central pain. Its pharmacological characteristic is being able to inhibit neuronal pre-synaptic membrane’s reuptake on 5-hydroxy trptamine and norepinephrine but instead have a low inhibitory effect on the reuptake of dopamine. Duloxetine hydrochloride is indicated for depression and it is effective in treating both endogenous and non-endogenous depression as well as the feeling of pain associated with depression. It has a therapeutic dose of 60mg/d~120mg/d with a good security and fewer adverse reactions with common adverse reactions such as nausea, dry mouth, constipation, poor appetite, fatigue, sleepiness, and increased sweating. Another indication is the pain caused by diabetic neuropathy.
An antidepressant. A dual serotonin and norepinephrine reuptake inhibitor (SNRI). Used in treatment of stress urinary incontinence.
1. Duloxetine Hydrochloride can inhibit neuron to 5 a serotonin and norepinephrine reuptake, thus raising the two central neurotransmitter concentrations in the brain and spinal cord.
2. Duloxetine Hydrochloride can be used to treat some mood disorders such as depression and anxiety symptoms and alleviate central pain such as diabetic peripheral neuropathic pain and women fibromyalgia, etc.
3. Duloxetine Hydrochloride can also be applied to the urethra serotonin and norepinephrine receptors, resulting in increased urethral sphincter nervous tension and contraction ability, so the stress urinary incontinence disease treatment is effective for women.