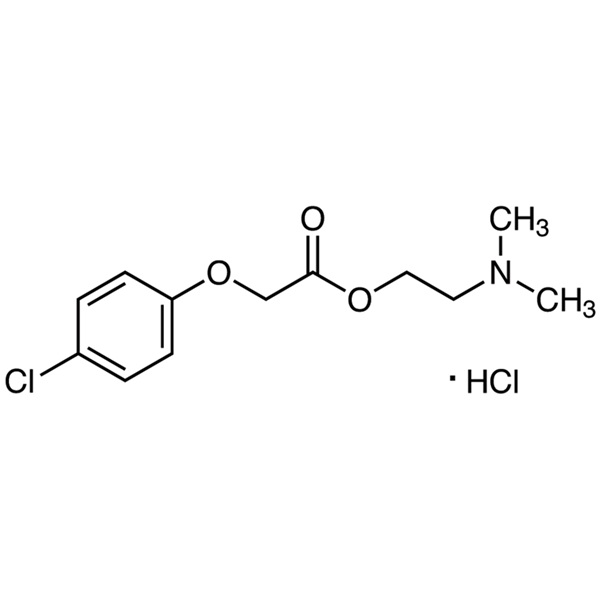
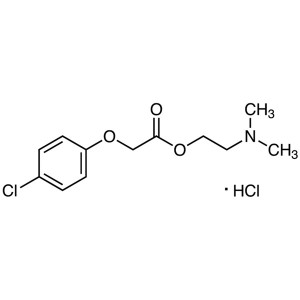
Meclofenoxate Hydrochloride CAS 3685-84-5 Assay >99.0% (HPLC) Nootropic
Ruifu Chemical is the leading manufacturer of Meclofenoxate Hydrochloride (Centrophenoxine Hydrochloride) (CAS: 3685-84-5) with high quality. Ruifu can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Meclofenoxate Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Meclofenoxate Hydrochloride |
| Synonyms | Centrophenoxine Hydrochloride; Meclofenoxate HCl; Centrophenoxine HCl; 2-(Dimethylamino)ethyl 2-(4-Chlorophenoxy)acetate Hydrochloride; 2-(4-Chlorophenoxy)acetic Acid 2-(Dimethylamino)ethyl Ester Hydrochloride |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 3685-84-5 |
| Molecular Formula | C12H16ClNO3·HCl |
| Molecular Weight | 294.17 g/mol |
| Melting Point | 139.0~143.0℃ |
| Sensitive | Hygroscopic, Heat Sensitive |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystals or Crystalline Powder | Conforms |
| Solubility | Freely soluble in water and in ethanol, sparingly soluble in acetic anhydride, and practically insoluble in diethyl ether. |
Conforms |
| Melting Point | 139.0~143.0℃ | 141.3℃ |
| Clarity and Color of Solution | Solution is Clear and Colorless | Conforms |
| Sulfate | ≤0.048% | < 0.048% |
| Heavy Metals | ≤20ppm | <10ppm |
| Arsenic | ≤2ppm | <2ppm |
| Organic Acids | Should Conform to Standards | Conforms |
| Water by Karl Fischer | ≤0.50% | 0.23% |
| Residue on Ignition | ≤0.10% | 0.04% |
| Assay / Analysis Method | >99.0% (HPLC) | 99.3% |
| pH | 3.5~4.5 | 4.2 |
| Infrared Spectrum | Conforms to Structure | Conforms |
| NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Acidity
Take 0.20g of this product and add 20ml of water to dissolve. The pH value should be 3.5~4.5 (General rule 0631).
Clarity of the solution
Take 1.0g of this product, add 10ml of water to dissolve, the solution should be clarified. (For injection)
Sulfate
Take 1.0g of this product and check it according to law (General rule 0802). Compared with the control solution made of 4.8ml of standard potassium sulfate solution, it should not be more concentrated (0.048%).
Organic Acids
Take this product 2.0g, add ether 50ml, shake for 10 minutes. Filter with G3 vertical melting funnel, the residue is washed with diethyl ether 2 times, each time 5ml, wash and filtrate, add neutral ethanol 50ml and phenolphthalein indicator solution 5 drops, with sodium hydroxide titration solution (0.1mol/L) titration, consumption of sodium hydroxide titration solution (0.1mol/L) not over 0.54.
Related Substances
New system for clinical use. Take about 50mg of this product, weigh it accurately, put it in a 50ml measuring flask, add an appropriate amount of solvent [water (adjust pH to 2.5 with phosphoric acid)-acetonitrile (4060)], shake to dissolve, and diluted to the scale, shake, as a test solution; Precision take 1ml, 100ml flask, diluted with the above solvent to the scale, shake, as a control solution. Test according to high performance liquid chromatography (General 0512). Silica gel bonded with eighteen alkyl silane was used as the filler, and 0.05mol/L sodium octane sulfonate (adjusted to pH 2.5 with phosphoric acid)-acetonitrile (65:35) was used as the mobile phase, and the detection wavelength was 225nm. Take about 10mg of this product, put it in a 10ml measuring flask, add 4ml of water to dissolve, heat it in a water bath for 5 minutes, dilute it with acetonitrile to the scale, shake well, and inject l0ul into the liquid chromatograph, the degree of separation of the meclofenoxate peak from the hydrolysis product peak (relative retention time about 0.6) should be greater than 6.0.1 Ou1 of the test solution and the control solution were respectively injected into the liquid chromatograph, and the chromatogram was recorded to 2 times of the retention time of the main component peak. If there are impurity peaks in the chromatogram of the test solution, the area of a single impurity peak shall not be greater than 0.5 times (0.5%) the area of the main peak of the control solution, and the sum of the areas of each impurity peak shall not be greater than the area of the main peak of the control solution (1.0%).
Loss on Drying
Take this product, dry to constant weight at 105℃, weight loss should not exceed 1.0% (for oral use) or 0.5% (for injection) (General 0831).
Ignition Residue
Not more than 0.1% (General rule 0841).
Heavy Metals
Take 1.0g of this product, add 23ml of water to dissolve, add 2ml of acetate buffer (pH 3.5), and check according to law (General rule 0821 first law), containing heavy metals shall not exceed 10 parts per million.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xn - Harmful
Risk Codes
R22 - Harmful if swallowed
R36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
RTECS AG0440000
Meclofenoxate Hydrochloride (Centrophenoxine Hydrochloride) (CAS: 3685-84-5) has been shown to improve memory, have a mentally stimulating effect, and improve general cognition. Meclofenoxate hydrochloride is a psychostimulant in the nootropic agent group available in capsule and tablet formulations approved for traumatic cataphora, alcoholic poisoning, anoxia neonatorum, and children's enuresis in China. Although these 2 generic formulations are marketed in China, information regarding their pharmacokinetics and bioequivalence in humans has not been published. Meclofenoxate hydrochloride appears to increase the consolidation of new information into long-term memory. Meclofenoxate does not affect other aspects of remembering. Meclofenoxate is found that significantly more of the subjects receiving meclofenoxate reportes an increased level of mental alertness.
Meclofenoxate hydrochloride is a cholinergic nootropic used as a dietary supplement and drug in the treatment of symptoms of senile dementia and Alzheimer's disease.
Meclofenoxate Hydrochloride is an anti-aging drug used to treat senile dementia and Alzheimer's disease. It can also inhibit the activity of cholinephosphotransferase. Meclofenoxate Hydrochloride is a nootropic medication offering fantastic supplementation of choline. Meclofenoxate Hydrochloride is one of the oldest and best studied nootropics and is well established as a great way of enhancing cognitive ability.
Used as a central nervous stimulant, it mainly acts on the cerebral cortex. It can promote the redox of brain cells, regulate the metabolism of nerve cells, increase the use of carbohydrates and have an excitatory effect on the inhibited central nervous system. Traumatic coma, childhood enuresis, disturbance of consciousness, senile psychosis, various dementias, alcoholism, etc.
The applications of is Meclofenoxate hydrochloride:
Memory decline through aging
Dementia and Alzheimer’s (clinically)
Brain damage or injuries
Alcohol or drug abuse symptoms and effects
Promote the metabolism of brain