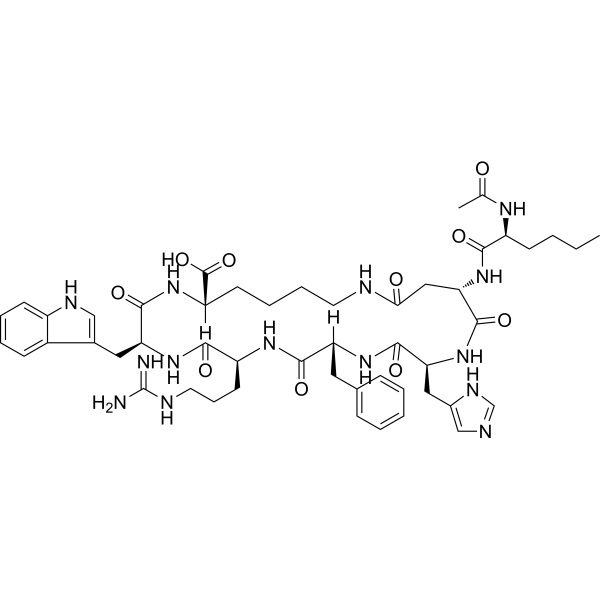
Bremelanotide (PT-141) CAS 189691-06-3 Purity ≥99.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Bremelanotide (PT-141) (CAS: 189691-06-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Bremelanotide (PT-141), Please contact: alvin@ruifuchem.com
| Chemical Name | Bremelanotide (PT-141) |
| Sequence | Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH |
| Synonyms | PT141; N-Acetyl-L-norleucyl-L-alpha-aspartyl-L-histidyl-D-phenylalanyl-L-arginyl-L-tryptophyl-L-lysine (2-7)-lactam |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 189691-06-3 |
| Molecular Formula | C50H68N14O10 |
| Molecular Weight | 1025.16 g/mol |
| Density | 1.43 g/ml |
| COA & MSDS | Available |
| HPLC Result | Enclosed |
| Mass Spec. | Enclosed |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications |
| Appearance | White Powder |
| Amino Acid Composition | ±10% of Theoretical |
| Purity (HPLC) | ≥99.0% (HPLC) |
| Single Impurity (HPLC) | ≤1.00% |
| Water Content (Karl Fischer) | ≤6.00% |
| Acetate Content (HPIC) | ≤15.00% |
| Mass Spectrum | In Accordance with the Standard |
| IR Spectrum | In Accordance with the Standard |
| Conclusion | The product has been tested and complies with the given specifications |
| Note | All peptides are shipped as lyophilized powder. It’s for research use only. |
The products will be supplied to some countries in which it could be in conflict with existing patents. However the final responsibility lies exclusively with the buyer.
The products are for research or lab use only, not intended for human use.
HPLC Result as Follows:
Project Name: PT-141 Processing Method: G.E
Injection Volume: 20.00ul Run Mode: Analysis
Run Time: 30 Minutes Lambda: 220nm
Peak Results
Peak No Ret time Peak Height Peak Area Result
1 14.910 2842.589 24279.842 0.4177
2 15.140 534150.625 5768839.500 99.2340
3 17.172 2670.344 20248.998 0.3483
539663.558 5813368.340 100.0000
Method Information
Merck (250×4.6mm I.D.) C18 Buffer A: 0.10% TFA+CH3CN
Flow Rate: 1.0ml/min Buffer B: 0.10% TFA+H2O
Gradient: 30%-60%A in 0-30 minutes
Package: Bottle, vial, vacuum packing. Accurate to the milligram as required.
Storage: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. And avoid frequent thawing and freezing.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Bremelanotide (PT-141) (CAS: 189691-06-3) was approved by the FDA in June 2019 for the treatment of acquired, generalized HSDD in premenopausal women. Bremelanotide activates melanocortin receptors,but the mechanism by which it improves sexual desire is unknown. To use bremelanotide, women inject it under the skin ofthe abdomen or thigh at least 45 minutes before anticipated sexual activity.The optimal time to inject bremelanotide may vary based on the duration of benefit and side effects experienced. More than one dose of bremelanotide should not be used within 24 hours or more than eight doses per month. Common side effects include nausea, vomiting, flushing, injection site reactions, and headache. Bremelanotide should not be used in women with high blood pressure that is uncontrolled or in those with known cardiovascular disease, and it is not recommended for women at high risk for cardiovascular disease. The safety and efficacy of bremelanotide has not been studied in breast cancer survivors, and there are no recommendations regarding its use in this population.
-
Bremelanotide (PT-141) CAS 189691-06-3 Purity ≥...
-
Cetrorelix Acetate CAS 130143-01-0 GnRH Antagon...
-
Deslorelin Acetate CAS 57773-65-6 GnRH Agonist ...
-
Desmopressin Acetate CAS 16789-98-3 Peptide Pur...
-
Melanotan II (MT-II) CAS 121062-08-6 Peptide Pu...
-
Octreotide Acetate CAS 83150-76-9 Peptide Purit...
-
Histrelin Acetate CAS 76712-82-8 Peptide Purity...
-
Eptifibatide Acetate CAS 148031-34-9 (Free Base...
-
Goserelin Acetate CAS 145781-92-6 Purity >99.0%...