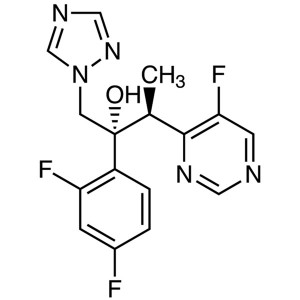
Voriconazole CAS 137234-62-9 Assay 97.5~102.0% Antifungal
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Voriconazole (CAS: 137234-62-9) with high quality, Antifungal. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Voriconazole, Please contact: alvin@ruifuchem.com
| Chemical Name | Voriconazole |
| Synonyms | UK-109496; (2R,3S)-2-(2,4-Difluorophenyl)-3-(5-Fluoro-4-Pyrimidinyl)-1-(1H-1,2,4-Triazol-1-yl)-2-Butanol; (2R,3S)-2-(2,4-Difluorophenyl)-3-(5-Fluoropyrimidin-4-yl)-1-(1,2,4-Triazol-1-yl)butan-2-ol |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 137234-62-9 |
| Molecular Formula | C16H14F3N5O |
| Molecular Weight | 349.32 g/mol |
| Melting Point | 130.0 to 134.0℃ |
| Specific Rotation [a]20/D | -58.0° to -62.0° (C=1, MeOH) |
| Density | 1.42±0.10 g/cm3 |
| Water Solubility | Insoluble Soluble in Water, Soluble in Methanol |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Shelf Life | Limited Shelf Life, Expiry Date on the Label |
| Product Categories |
API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Powder | White Powder |
| Melting Point | 130.0 to 134.0℃ | 132.5℃ |
| Heavy Metals | ≤10ppm | <10ppm |
| Water by Karl Fischer | ≤0.40% | <0.40% |
| Residue on Ignition | ≤0.10% | <0.10% |
| Related Substances | ||
| Voriconazole Related Compound C | ≤0.20% | <0.20% |
| Voriconazole Related Compound D | ≤0.10% | <0.10% |
| Voriconazole Related Compound F | ≤0.10% | <0.10% |
| Voriconazole Related Compound B | ≤0.20% | <0.20% |
| Any Unspecified Impurity | ≤0.10% | <0.10% |
| Total impurities | ≤0.50% | <0.50% |
| Voriconazole Assay | 97.5~102.0% | Conforms |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Conclusion | Has been tested and complies with the USP35 specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Voriconazole
C16H14F3N5O 349.31
4-Pyrimidineethanol, α-(2,4-difluorophenyl)-5-fluoro-β-methyl-α-(1H-1,2,4-triazol-1-ylmethyl)-, (αR,βS)-;
(αR,βS)-α-(2,4-Difluorophenyl)-5-fluoro-β-methy-α-(1H-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol
[137234-62-9].
DEFINITION
Voriconazole contains NLT 97.5% and NMT 102.0% of Voriconazole (C16H14F3N5O), calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
A. INFRARED ABSORPTION <197K>
B. The retention time of the major peak of the Sample solution corresponds to that of System suitability solution A, as obtained in the test for Voriconazole Related Compound B.
ASSAY
PROCEDURE
Buffer: 1.9 g/L of ammonium formate in water. Adjust with formic acid to a pH of 4.0.
Mobile phase: Acetonitrile, methanol, and Buffer (15:30:55)
Standard solution: 25 μg/mL of USP Voriconazole RS in Mobile phase. [NOTE-Sonicate to dissolve, if necessary.]
Sample solution: 25 μg/mL of Voriconazole in Mobile phase. [NOTE-Sonicate to dissolve, if necessary.]
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 256 nm
Column: 3.9-mm × 15-cm; 4-μm packing L1
Column temperature: 35°
Flow rate: 1 mL/min
Injection size: 20 μL
System suitability
Sample: Standard solution
Tailing factor: NMT 2.0
Column efficiency: NLT 3500 theoretical plates
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of voriconazole (C16H14F3N5O) in the portion of Voriconazole taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Voriconazole RS in the Standard solution (μg/mL)
CU = concentration of voriconazole in the Sample solution (μg/mL)
Acceptance criteria: 97.5%~102.0% on the anhydrous and solvent-free basis
IMPURITIES
HEAVY METALS, Method II <231>: NMT 10 ppm
RESIDUE ON IGNITION <281>: NMT 0.1%
VORICONAZOLE RELATED COMPOUNDS C AND D
Mobile phase and Chromatographic system: Proceed as directed in the Assay
System suitability solution: 0.25 μg/mL of USP Voriconazole RS
Standard solution: 2.5 µg/mL each of USP Voriconazole RS, USP Voriconazole Related Compound C RS, and USP Voriconazole Related Compound D RS in Mobile phase. [NOTE-Sonicate to dissolve, if necessary.]
Sample solution: 500 µg/mL of Voriconazole in Mobile phase. [NOTE-Sonicate to dissolve, if necessary.]
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Tailing factor: NMT 2.0 for the voriconazole peak, Standard solution
Column efficiency: NLT 3500 theoretical plates for the voriconazole peak, Standard solution
Relative standard deviation: NMT 10.0%, System suitability solution
Analysis
Samples: Standard solution and Sample solution Calculate the percentage of voriconazole related Voriconazole Related Compound F RS. Dissolve in 50% compound C and voriconazole related compound D in the portion of Voriconazole taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of Voriconazole related compound C or voriconazole related compound D from the Sample solution
rS = peak response of Voriconazole related compound C or voriconazole related compound D from the Standard solution
CS = concentration of USP Voriconazole Related Compound C RS or USP Voriconazole Related Compound D RS in the Standard solution (µg/mL)
CU = concentration of Voriconazole in the sample solution (µg/mL)
Calculate the percentage of any unspecified impurity in the portion of Voriconazole taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of any individual impurity from the sample solution
rS = peak response of Voriconazole from the standard solution
CS = concentration of USP Voriconazole RS in the standard solution (µg/mL)
CU = concentration of Voriconazole in the sample solution (µg/mL)
Acceptance criteria: See Table 1.
Table 1
Name Relative Retention Time Acceptance Criteria, NMT (%)
Voriconazole related compound Ca 0.26 0.2
Voriconazole related compound Db 0.61 0.1
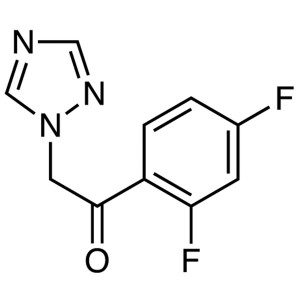
a 1-(2,4-Difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone
b (2RS,3SR)-2-(2,4-Difluorophenyl)-3-(pyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol.
c Disregard peaks less than 0.05%
d Include Voriconazole related compound B and Voriconazole related compound F.
Table 1 (Continued)
Name Relative Retention Time Acceptance Criteria, NMT (%)
Voriconazole 1.0 -
Any unspecified impurityc - 0.1
Total impuritiesd - 0.5
a 1-(2,4-Difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone.
b (2RS,3SR)-2-(2,4-Difluorophenyl)-3-(pyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol.
c Disregard peaks less than 0.05%
d Include Voriconazole related compound B and voriconazole related compound F.
VORICONAZOLE RELATED COMPOUND F
Sodium hydroxide solution: 470 g/L of sodium hydroxide in water
Mobile phase: Methanol, water, and Sodium hydroxide solution (500: 1500: 0.175). [NOTE-Minimize the carbonate formation in the Mobile phase by degassing methanol and water before mixing.]
Suppressant solution: 12 mM of sulfuric acid in water
Chloride stock solution: 85 µg/mL of sodium chloride in water
Standard stock solution: 250 µg/mL of USP
Voriconazole Related Compound F RS. Dissolve in 50% of the final volume with methanol, and dilute with Mobile phase to volume.
Standard solution: 5 µg/mL of USP Voriconazole Related Compound F RS from the Standard stock solution in a mixture of methanol and Mobile phase (50:50)
System suitability solution A: 5 µg/mL of USP Voriconazole Related Compound F RS from the Standard stock solution and 1.7 µg/mL of USP Sodium Chloride RS in a mixture of methanol and Mobile phase (50:50)
System suitability solution B: 2.5 µg/mL of USP Voriconazole Related Compound F RS from the Standard solution in Mobile phase
Sample solution: 5 mg/mL of Voriconazole. Dissolve in 50% of the final volume with methanol, and dilute with Mobile phase to volume
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: Ion chromatography/LC
Detector: Conductivity with anion suppressor
Column: 4-mm × 5-cm guard column and 4-mm × 25-cm analytical column; both packing L46
Column temperature: 40°
Flow rate: 1 mL/min
Flow rate (for anion suppressor): 2 mL/min
Injection size: 20 µL
System suitability
Samples: System suitability solution A and System suitability solution B
[NOTE-The relative retention times for acetate ion (for information only), Voriconazole related compound F, and chloride ion are 0.47, 1.0, and 1.5, respectively.]
Suitability requirements
Resolution: NLT 3.5 between the Voriconazole related compound F and chloride peaks, System suitability solution A
Tailing factor: NMT 2.0, System suitability solution B
Relative standard deviation: NMT 10.0%, System suitability solution B
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of Voriconazole related compound F in the portion of Voriconazole taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of Voriconazole related compound F from the Sample solution
rS = peak response of voriconazole related compound F from the Standard solution
CS = concentration of USP Voriconazole Related Compound F RS in the Standard solution (µg/mL)
CU = concentration of voriconazole in the Sample solution (µg/mL)
Acceptance criteria: NMT 0.1%
VORICONAZOLE RELATED COMPOUND B
Initially dissolve the Standard and sample materials in 4% of the final volume of acetonitrile.
Buffer: 0.8 g/L of ammonium acetate. Adjust with glacial acetic acid to a pH of 5.0.
Mobile phase: Acetonitrile and Buffer (18:82)
System suitability solution A: 500 µg/mL of USP Voriconazole RS and 2.5 µg/mL of USP Voriconazole Related Compound B in Mobile phase
System suitability solution B: 0.25 µg/mL of USP Voriconazole Related Compound B RS in Mobile phase
Standard solution: 2.5 µg/mL of USP Voriconazole Related Compound B RS in Mobile phase
Sample solution: 500 µg/mL of Voriconazole in Mobile phase
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 256 nm
Column: 4.6-mm × 25-cm; 5-µm packing L45
Column temperature: 30°
Flow rate: 1 mL/min
Injection size: 20 µL
System suitability
Samples: System suitability solution A and System suitability solution B
[NOTE-The relative retention times for Voriconazole and Voriconazole related compound B are 1.0 and 1.4, respectively.]
Suitability requirements
Resolution: NLT 4.0 between the Voriconazole and Voriconazole related compound B peaks, System suitability solution A
Tailing factor: NMT 2.0 for the Voriconazole related compound B peak, System suitability solution A
Relative standard deviation: NMT 10.0%, System suitability solution B
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of voriconazole related compound B in the portion of Voriconazole taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of Voriconazole related compound B from the Sample solution
rS = peak response of Voriconazole related compound B from the Standard solution
CS = concentration of USP Voriconazole Related Compound B RS in the Standard solution (µg/mL)
CU = concentration of voriconazole in the Sample solution (µg/mL)
Acceptance criteria: NMT 0.2%
SPECIFIC TESTS
BACTERIAL ENDOTOXINS TEST <85>: Where the label states that Voriconazole is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.2 USP Endotoxin Units/mg of voriconazole.
STERILITY TESTS <71>: Where the label states that Voriconazole is sterile, it meets the requirements.
WATER DETERMINATION, Method I <921>: NMT 0.4%
ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers, and store at room temperature.
LABELING: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms
USP REFERENCE STANDARDS <11>
USP Endotoxin RS
USP Voriconazole RS
USP Voriconazole Related Compound B RS
(2S,3R)-2-(2,4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol.
C16H14F3N5O 349.31
USP Voriconazole Related Compound C RS
1-(2,4-Difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone
C10H7N3OF2 223.18
USP Voriconazole Related Compound D RS
(2RS,3SR)-2-(2,4-Difluorophenyl)-3-(pyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol.
C16H15F2N5O 331.32
USP Voriconazole Related Compound F RS
{(1RS,4SR)-7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl}methanesulfonic acid.
C10H16O4S 232.30 2S (USP35)
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Hazard Codes | Xn,T,F |
| Risk Statements | 22-36/38-52/53-48/22-40-25-61-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-45-36/37-22-53-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | UV9145000 |
| Hazard Class | 6.1 |
| Packing Group | III |
| HS Code | 2934999099 |
Voriconazole (CAS: 137234-62-9) is a broad-spectrum triazole antifungal ,it is primarily used for the treatment of progressive, possibly life-threatening infections in immune deficiency patients. Indications include: immunosuppressed patients with severe fungal infections, acute invasive aspergillosis (the most common pathogen is Aspergillus fumigatus, followed by A. flavus, Aspergillus niger and Aspergillus soil), severe invasive infections caused by fluconazole-resistant Candida (including C. krusei) severe infection caused by Foot actinomycetes bacteria genu. Voriconazole was introduced in the US for the treatment of acute invasive aspergillosis, candidosis and other emerging fungal infections seen in immuno compromised patients.
1. Voriconazole is a triazole antifungal medication, it can cure empirical antifungal therapy.
2. Voriconazole has become the new standard of care in the treatment of invasive aspergillosis.
3. Voriconazole can used in the treat of esophageal candidiasis.
4. Voriconazole has also been used to treat severe fungal corneal infection.
5. An antifungal (systemic). An ergosterol biosynthesis inhibitor.