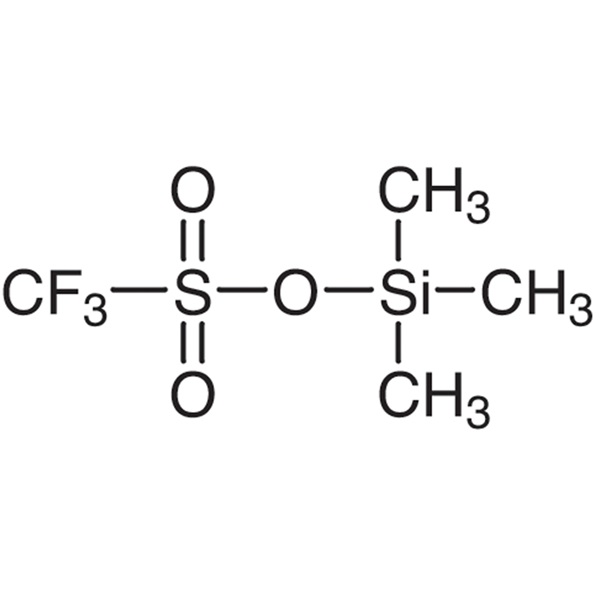
Trimethylsilyl Trifluoromethanesulfonate (TMSOTf) CAS 27607-77-8 Purity >99.0% (GC)
Ruifu Chemical is the leading manufacturer and supplier of Trimethylsilyl Trifluoromethanesulfonate (TMSOTf) (CAS: 27607-77-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase TMSOTf, Please contact: alvin@ruifuchem.com
| Chemical Name | Trimethylsilyl Trifluoromethanesulfonate |
| Synonyms | TMS Triflate; TMSOTf; Trifluoromethanesulfonic Acid Trimethylsilylester; Trimethylsilyl Triflate; Trifluoromethanesulfonic Acid Trimethylsilyl Ester; TMS-OTf |
| CAS Number | 27607-77-8 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C4H9F3SiSO3 |
| Molecular Weight | 222.26 |
| Boiling Point | 77℃/80 mmHg (lit.) |
| Flash Point | 25℃ |
| Density | 1.228 g/mL at 25℃(lit.) |
| Refractive Index n20/D | 1.36(lit.) |
| Form | Fuming Liquid |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitivity | Moisture & Air Sensitive |
| Water Solubility | Reacts |
| Hydrolytic Sensitivity | 8: Reacts Rapidly With Moisture, Water, Protic Solvents |
| Hazard Class | 8 (3); Corrosive, Flammable Liquid |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless or Light Yellow Transparent Liquid, Stimulating Smell |
| Purity / Analysis Method | >99.0% (GC) |
| Proton NMR Spectrum | Conforms to Structure |
| Fluorine NMR Spectrum | Conforms to Structure |
| Density (20℃) | 1.228~1.230 |
| Refractive Index n20/D | 1.359~1.361 |
| H2O | ≤0.02% |
| SO42- | ≤50ppm |
| F- | ≤10ppm |
| Cl- | ≤50ppm |
| Test Standard | Enterprise Standard |
Package: Fluorinated Bottle, 25kg/Drum, 200kg/Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Avoid exposure to sunlight; avoid fire; avoid moisture. After the container is opened, it should be sealed to prevent water vapor from entering to produce hydrolysis.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.

Risk Codes R10 - Flammable
R14 - Reacts violently with water
R34 - Causes burns
Safety Description S16 - Keep away from sources of ignition.
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S8 - Keep container dry.
UN IDs UN 2920 8/PG 2
WGK Germany 3
FLUKA BRAND F CODES 10-21
TSCA Yes
HS Code 2903599000
Hazard Note Corrosive / Flammable
Hazard Class 3
Packing Group III
Trimethylsilyl Trifluoromethanesulfonate (TMSOTf) (CAS: 27607-77-8) is a strong Lewis acid catalyst and efficient silylating agent. Usually used in a Dieckmann-like cyclization of ester-imides and diesters,and also used to prepare difluoroboron triflate etherate a powerful Lewis acid especially in acetonitrile solvent. Trimethylsilyl Trifluoromethanesulfonate is generally used following reactions:1. Silylation. TMSOTf is widely used in the conversion of carbonyl compounds to their enol ethers. The conversion is some 109 faster with TMSOTf/triethylamine than with chlorotrimethylsilane. Trimethylsilyl Trifluoromethanesulfonate is used as catalyst and silicon based reagent in organic synthesis
Trimethylsilyl Trifluoromethanesulfonate has been used in combination with boron trifluoride etherate for the copper-catalyzed asymmetric allylic alkylation (AAA) of allyl bromides, chlorides, and ethers with organolithium reagents in the presence of a chiral ligand.
Trimethylsilyl Trifluoromethanesulfonate is a useful reagent in organic synthesis. It is a strong nucleophile which can be used as a catalyst in organic reactions. TMSOTf is commonly used in the synthesis of pharmaceuticals, agrochemicals, and other industrial chemicals. TMSOTf is also used in the synthesis of peptides, peptidomimetics, and other biomolecules. Additionally, it contributes to the synthesis of polymers, dyes, and diverse materials. With its potent nucleophilicity, TMS-TFMS effectively reacts with electrophilic compounds, encompassing alkenes, alkynes, aromatics, and heterocycles. The reagent′s ability to form covalent bonds with electrophiles leads to the creation of novel molecules, further enhancing its utility in organic synthesis.
-
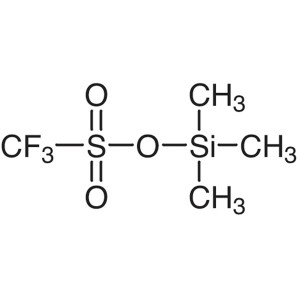
Trimethylsilyl Trifluoromethanesulfonate (TMSOT...
-
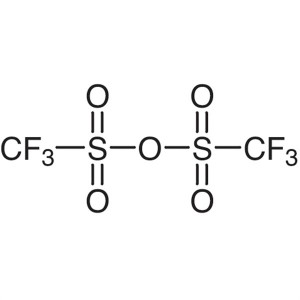
Trifluoromethanesulfonic Anhydride CAS 358-23-6...
-
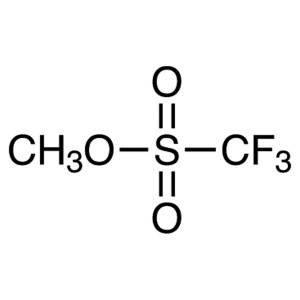
Methyl Trifluoromethanesulfonate CAS 333-27-7 P...
-
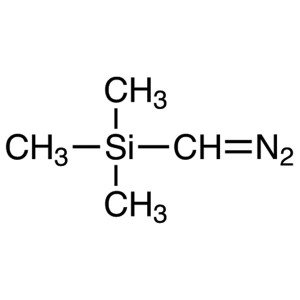
(Trimethylsilyl)diazomethane CAS 18107-18-1 2.0...
-
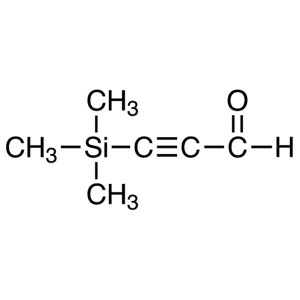
3-Trimethylsilylpropynal CAS 2975-46-4 Purity >...
-
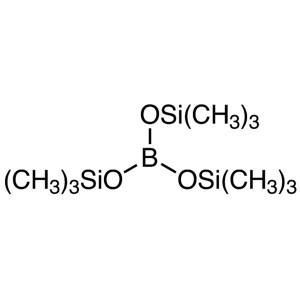
Tris(trimethylsilyl) Borate (TMSB) CAS 4325-85-...
-
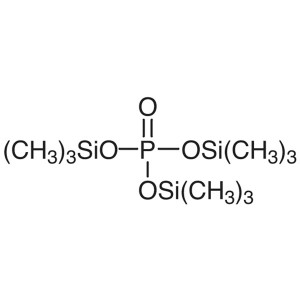
Tris(trimethylsilyl) Phosphate (TMSP) CAS 10497...
-
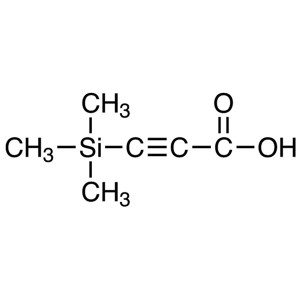
3-(Trimethylsilyl)propiolic Acid CAS 5683-31-8 ...
-
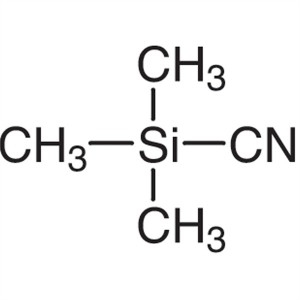
Trimethylsilyl Cyanide TMSCN CAS 7677-24-9 Assa...
-
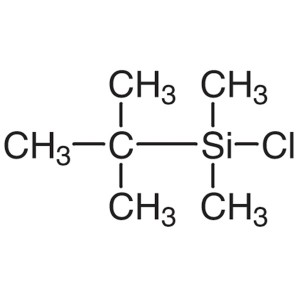
TBDMSCl CAS 18162-48-6 tert-Butyldimethylsilyl ...
-
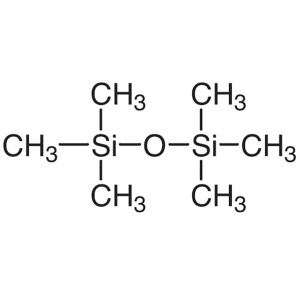
Hexamethyldisiloxane (HMDSO) CAS 107-46-0 Purit...
-
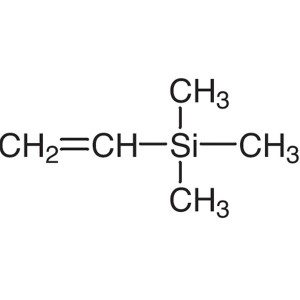
Vinyltrimethylsilane CAS 754-05-2 Purity >98.0%...
-
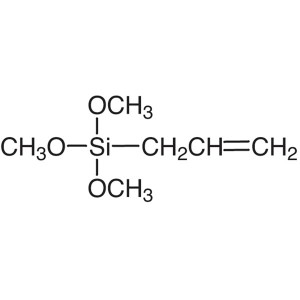
Allyltrimethoxysilane Trimethoxyallylsilane CAS...
-
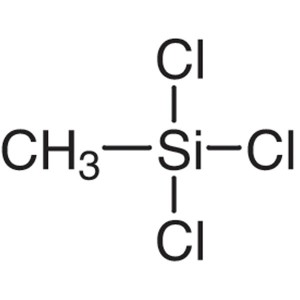
Methyltrichlorosilane CAS 75-79-6 Purity >99.0%...
-
tert-Butyldimethylsilane CAS 29681-57-0 Purity ...
-
Triethylsilane (TES) CAS 617-86-7 Purity >99.0%...