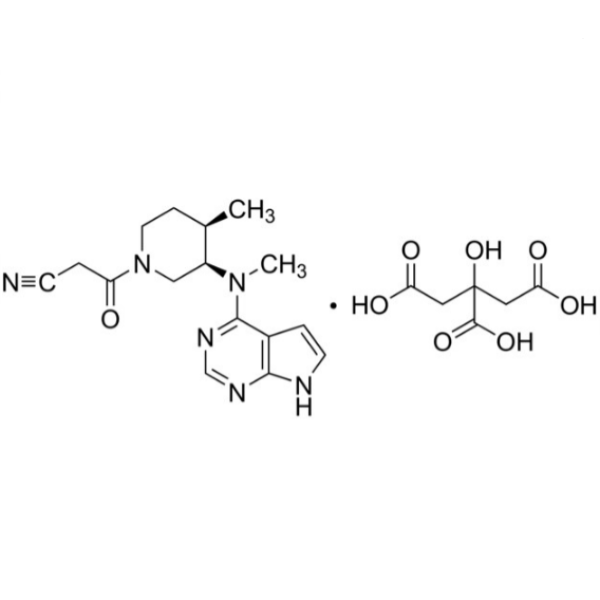
Tofacitinib Citrate CAS 540737-29-9 Assay 98.0~101.0%
Ruifu Chemical is the leading manufacturer of Tofacitinib Citrate (CAS: 540737-29-9) with high quality, API, used for treating rheumatoid arthritis. Ruifu Chemical has been supplying pharmaceutical intermediates and APIs, fine chemicals more than 15 years. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service. Purchase Tofacitinib Citrate or other products, Please contact: alvin@ruifuchem.com
| Chemical Name | Tofacitinib Citrate |
| Synonyms | Tasocitinib Citrate Salt; CP-690550 Citrate; (3R,4R)-4-Methyl-3-(Methyl-7H-Pyrrolo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-1-Piperidinepropanenitrile Citrate Salt; 3-[(3R,4R)-4-Methyl-3-[Methyl-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-Oxopropanenitrile Citrate Salt |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 540737-29-9 |
| Molecular Formula | C16H20N6O.C6H8O7 |
| Molecular Weight | 504.497 g/mol |
| Melting Point | 201℃(Decomp) |
| COA & MSDS | Available |
| Place of Origin | Shanghai, China |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Caution | For Research Use Only, Not for Human or Diagnostic Use |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Powder | White Powder |
| Solubility | Freely Soluble in Dimethyl Sulfoxide, Slightly Soluble in Water, Practically Insoluble in, or Insoluble in Ethanol | Conforms |
| Identification | The retention time of the major peak in the sample solution corresponds to that of the Tofacitinib from the System suitability solution in the test for Enantiomer | Conforms |
| IR: The spectrum of the sample is consistent with that of the reference standard spectrum |
Conforms | |
| Citrate: Add about 13mg to a mixture of 1ml of acetic anhydride and 3ml of pyridine. A yellow to red or purplish red colour develops. | Generate Yellow Solution | |
| Loss on Drying | ≤0.50% | 0.16% |
| Residue on Ignition | ≤0.10% | 0.02% |
| Chloride | ≤0.01% | <0.01% |
| Relates Substances | N.D. | |
| Tofacitinib Impurity-24 | ≤0.10% | 0.03% |
| Tofacitinib Impurity-05 | ≤0.10% | N.D. |
| Tofacitinib Impurity-12 | ≤0.10% | 0.02% |
| Tofacitinib Impurity-06 | ≤0.10% | N.D. |
| Tofacitinib Impurity-07 | ≤0.15% | N.D. |
| Tofacitinib Impurity-02 | ≤0.10% | N.D. |
| Tofacitinib Impurity-10 | ≤0.10% | 0.02% |
| Tofacitinib Impurity-36 | ≤0.15% | 0.08% |
| Largest Unknown Impurity | ≤0.10% | 0.02% (RRT 1.452) |
| Total Impurities | ≤0.50% | 0.22% |
| Enantiomer Tofacitinib Impurity-01 | ≤0.10% | N.D. |
| Residual Solvents 1 | ||
| Ethyl Acetate | ≤0.50% | N.D. |
| Benzene | ≤0.0002% | N.D. |
| 2-Methyltetrahydrofuran | ≤0.062% | <0.001% |
| Dichloromethane | ≤0.06% | N.D. |
| Tetrahydrofuran | ≤0.072% | N.D. |
| Residual Solvents 2 | ||
| Methylamine | ≤0.10% | N.D. |
| Methanol | ≤0.30% | N.D. |
| Ethanol | ≤0.50% | 0.31% |
| Isopropylamine | ≤0.01% | N.D. |
| Methyl Tert-butyl Ether | ≤0.50% | N.D. |
| Butyl Alcohol | ≤0.50% | 0.01% |
| n-Heptane | ≤0.50% | N.D. |
| Toluene | ≤0.089% | N.D. |
| Residual Solvents 3 | ||
| Formic Acid | ≤0.15% | N.D. |
| Acetic Acid | ≤0.15% | N.D. |
| Cyanoacetic Acid | ≤0.15% | 0.01% |
| Elemental Impurity | ||
| Pd | ≤10ppm | 0.1ppm |
| Microbiological Quality | ||
| Total Aerobic Microbial Count | ≤1000CFU/g | <10CFU/g |
| Mold & Yeast | ≤100CFU/g | <10CFU/g |
| Specified Microorganism | Absence of Escherichia Coil (1g) | N.D. |
| Assay | 98.0~101.0% (Calculated on Dried Basis) | 99.9% |
| Citric Acid | 34.3~41.8% (Calculated on Dried Basis) | 36.9% |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
None of the products will be supplied to countries in which this could be in conflict with the existing patents. However the final responsibility lies with the Buyer.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| WGK Germany | 3 |
| HS Code | 2933599099 |
Tofacitinib Citrate (CAS: 540737-29-9) is a king of drugs developed by the US pharmaceutical company Pfizer for treating rheumatoid arthritis, trade name Xeljanz, for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid arthritis (RA) in adult patients. This product is a Janus kinase inhibitor, administered twice daily. November 6, 2012, the US Food and Drug Administration (FDA) and Pfizer jointly announced Tofacitinib citrate is approved for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid joints arthritis (RA) in adult patients. Xeljanz can be used as monotherapy or in combination with methotrexate or other non-biological disease-modifying antirheumatic drugs (the DMARD) in combination. This medicine should not be in combination with biological DMARD or strong immunosuppressants (such as cyclosporine and azathioprine). Xeljanz is approved by the daily dose of 2 times, each time 5mg.
Tofacitinib Citrate can be used in combination for the treatment of active rheumatoid arthritis that has had an inadequate response to tumor necrosis factor inhibitors.
Tofacitinib Citrate is a king of drugs developed by the US pharmaceutical company for treating rheumatoid arthritis, for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid arthritis (RA) in adult patients. Tofacitinib Citrate is approved for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid joints arthritis (RA) in adult patients.
Tofacitinib Citrate can be used as monotherapy or in combination with methotrexate or other non-biological disease-modifying antirheumatic drugs (the DMARD) in combination. This medicine should not be in combination with biological DMARD or strong immunosuppressants (such as cyclosporine and azathioprine) . Tofacitinib Citrate is approved by the daily dose of 2 times, each time 5mg. Seven clinical trials evaluated the safety and efficacy of Tofacitinib Citrate in moderate to severe active RA in adult patients. In all tests, compared with patients receiving placebo, patients receiving Tofacitinib Citrate treatment showed significant improvement in clinical response and physical function.abbreviated as NAD+ and NADH respectively.
-
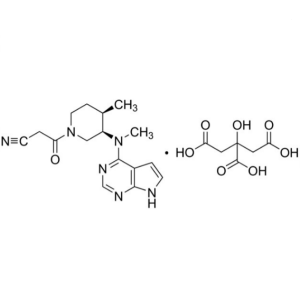
Tofacitinib Citrate CAS 540737-29-9 Assay 98.0~...
-
![4-Chloropyrrolo[2,3-d]pyrimidine CAS 3680-69-1 Purity >98.0% (HPLC) Tofacitinib Citrate Intermediate](https://www.ruifuchem.com/uploads/4-Chloropyrrolo23-dpyrimidine-CAS-3680-69-1-Tofacitinib-Citrate-Intermediate-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
4-Chloropyrrolo[2,3-d]pyrimidine CAS 3680-69-1 ...
-
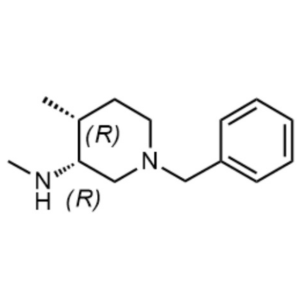
(3R,4R)-1-Benzyl-N,4-Dimethylpiperidin-3-Amine ...
-
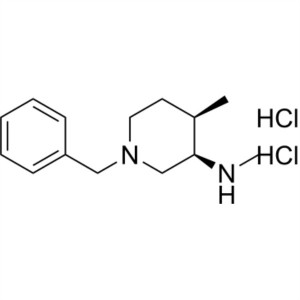
(3R,4R)-1-Benzyl-N,4-Dimethylpiperidin-3-Amine ...
-
![2,4-Dichloro-7H-Pyrrolo[2,3-d]pyrimidine CAS 90213-66-4 Purity >99.0% (HPLC) Tofacitinib Citrate Intermediate](https://www.ruifuchem.com/uploads/24-Dichloro-7H-Pyrrolo23-dpyrimidine-CAS-90213-66-4-Tofacitinib-Citrate-Intermediate-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
2,4-Dichloro-7H-Pyrrolo[2,3-d]pyrimidine CAS 90...
-
![4-Chloro-7-Tosyl-7H-Pyrrolo[2,3-d]pyrimidine CAS 479633-63-1 Purity >98.0% (HPLC) Tofacitinib Citrate Intermediate](https://www.ruifuchem.com/uploads/4-Chloro-7-Tosyl-7H-Pyrrolo23-dpyrimidine-CAS-479633-63-1-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)
4-Chloro-7-Tosyl-7H-Pyrrolo[2,3-d]pyrimidine CA...
-
Tofacitinib Citrate Intermediate CAS 477600-71-...