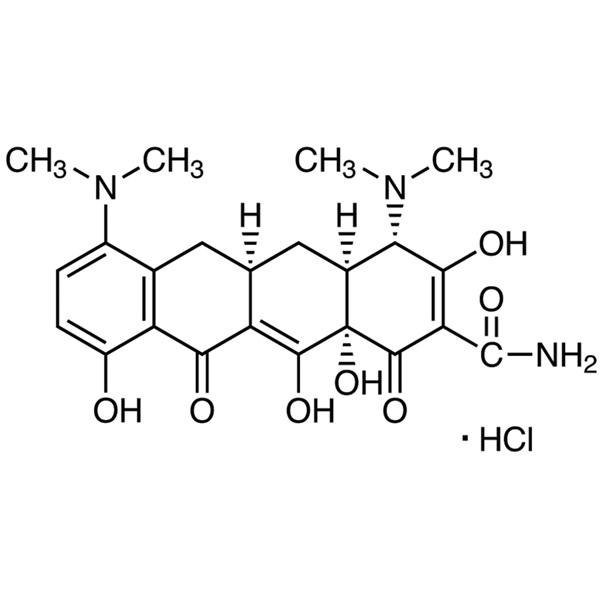
Minocycline Hydrochloride CAS 13614-98-7 Assay 890~950μg/mg
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Minocycline Hydrochloride (CAS: 13614-98-7) with high quality. Ruifu Chemical is committed to the manufacture and development of advanced, high quality pharmaceutical intermediates and APIs (Active Pharmaceutical Ingredients). Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Minocycline Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Minocycline Hydrochloride |
| Synonyms | Minocyclin HCl; [4S-(4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)- 1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a, tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide |
| Stock Status | In Stock, Production Capacity 20 Metric Tons per Year |
| CAS Number | 13614-98-7 |
| Molecular Formula | C23H27N3O7·HCl |
| Molecular Weight | 493.94 g/mol |
| Melting Point | 205.0~210.0℃(dec) |
| Boiling Point | 813℃ |
| Flash Point | >110℃(230°F) |
| Sensitive | Heat Sensitive |
| Water Solubility | Slightly Soluble in Water |
| Solubility | Soluble in Dimethylformamide, Methanol. Very Slightly Soluble in Ethanol |
| COA & MSDS | Available |
| Shelf Life | >2 Years if Stored Properly |
| Place of Origin | Shanghai, China |
| Product Categories |
API (Active Pharmaceutical Ingredient) |
| Caution | Not Intended for Human or Veterinary Use. For Research Use Only |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Yellow Crystalline Powder | Yellow Crystalline Powder |
| Crystallinity | Meet the Requirements | Complies |
| Identification | IR Similar to Standard | Complies |
| Acidity pH | 3.5~4.5 | 3.9 |
| Water by Karl Fischer | 4.3%~8.0% | 4.5% |
| Residue on Ignition | <0.15% | 0.07% |
| Heavy Metals (Pb) | <50ppm | <50ppm |
| Epi-Minocycline | <1.2% | 0.7% |
| Max. Individual Impurity | <1.2% | 0.2% |
| Total Impurities | <2.0% (Excluding Epiminocycline) | <2.0% |
| Assay / Analysis Method | 890μg/mg~950μg/mg (as C23H27N3O7 on Anhydrous Basis) |
929μg/mg |
| Residual Solvents | Methanol <0.3% | <0.3% |
| Particle Size | ≥250um: Less Than 10% | Complies |
| ≥150um: Less Than 40% | ||
| ≥38um: Less Than 50% | ||
| Conclusion | This product by inspection accords with the USP 35 standard | |
Package: Bottle, 1kg/Aluminum can, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃), well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Minocycline Hydrochloride
C23H27N3O7·HCl 493.94
2-Naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11dioxo-, monohydrochloride, [4S-(4,4a,5a,12a)]-;
4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride [13614-98-7].
DEFINITION
Minocycline Hydrochloride contains the equivalent of NLT 890 µg and NMT 950 µg of minocycline (C23H27N3O7) per mg, calculated on the anhydrous basis.
IDENTIFICATION
• Infrared Absorption <197K>: Dry the Standard and Sample at 100 for 2 h before use.
ASSAY
• Procedure
[Note-Protect the Standard solution and Sample solution from light, store in a refrigerator, and use within 3 h.]
Mobile phase: Dimethylformamide, tetrahydrofuran, 0.2 M ammonium oxalate, and 0.01 M edetate disodium (120:80:600:180). Adjust with ammonium hydroxide to a pH of 7.2.
System suitability solution: Dissolve 10 mg of USP Minocycline Hydrochloride RS in 20 mL of 0.2 M ammonium oxalate. Heat on a water bath at 60 for 3 h, allow to cool, and dilute with water to 25.0 mL.
Standard solution: Equivalent to 500 µg/mL of minocycline (C23H27N3O7) from USP Minocycline Hydrochloride RS in water
Sample solution: Equivalent to 500 µg/mL of minocycline (C23H27N3O7) from Minocycline Hydrochloride in water
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 40
Flow rate: 1.5 mL/min
Injection size: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[Note- The relative retention times for epiminocycline and minocycline are 0.7 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 4.6 between epiminocycline and minocycline, System suitability solution
Tailing factor: 0.9-2.0 for the analyte peak, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the quantity, in µg/mg, of minocycline (C23H27N3O7) in the portion of Minocycline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × P
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of minocycline in the Standard solution (µg/mL)
CU = concentration of the Sample solution (µg/mL)
P = potency of USP Minocycline Hydrochloride RS (µg/mg)
Acceptance criteria: 890-950 µg/mg on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.15%
• Heavy Metals, Method II <231>: NMT 50 ppm
Organic Impurities
• Procedure
Mobile phase and System suitability solution: Proceed as directed in the Assay
[Note-Protect the Sample solutions from light, store in a refrigerator, and use within 3 h.]
Sample solution 1: 0.25 mg/mL of Minocycline Hydrochloride
Sample solution 2: 5 µg/mL of Minocycline Hydrochloride in water
Sample solution 3: 3 µg/mL of Minocycline Hydrochloride in water
Chromatographic system: Proceed as directed in the Assay.
Run time: 2.6 times the retention time of minocycline, Sample solution 1
Analysis
Samples: Sample solution 1, Sample solution 2, and Sample solution 3
Calculate the percentage of epiminocycline in the portion of Minocycline Hydrochloride taken:
Result = (rE1/rM3) × D1 × 100
rE1 = peak response of epiminocycline from Sample solution 1
rM3 = peak response of minocycline from Sample solution 3
D1 = dilution factor for Sample solution 3
Calculate the total percentage of impurities other than epiminocycline in the portion of Minocycline Hydrochloride taken:
Result = (rT/rM2) × D2 × 100
rT = sum of peak responses of all impurities other than epiminocycline from Sample solution 1
rM2 = peak response of minocycline from Sample solution 2
D2 = dilution factor for Sample solution 2
Acceptance criteria
Individual impurities: NMT 1.2% epiminocycline
Total impurities (excluding epiminocycline): NMT 2.0%
SPECIFIC TESTS
• Crystallinity <695>: Meets the requirements
• pH <791>: 3.5-4.5, in a solution equivalent to 10 mg/mL of minocycline
• Water Determination, Method I <921>: 4.3%-8.0%
• Sterility Tests <71>: Where the label states that Minocycline Hydrochloride is sterile, it meets the requirements.
• Bacterial Endotoxins Test <85>: Where the label states that Minocycline Hydrochloride is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 1.25 USP Endotoxin Units/mg of minocycline.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers, protected from light.
• Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
• USP Reference Standards <11>
USP Endotoxin RS
USP Minocycline Hydrochloride RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
UN IDs 3249
WGK Germany 3
RTECS QI7630500
HS Code 2941302000
Hazard Class 6.1(b)
Packing Group III
Minocycline Hydrochloride (CAS: 13614-98-7) is a broad spectrum antibiotic with bacteriostatic function. Minocycline has anti-inflammatory properties.
Minocycline Hydrochloride is a tetracycline antibiotic that has been used in the treatment of various bacterial infections since its introduction in the 1970s. Minocycline Hydrochloride is a semisynthetic derivative of tetracycline and is structurally similar to other tetracycline antibiotics. The hydrochloride salt form of minocycline is the most commonly used form and is available in oral and intravenous formulations. Minocycline Hydrochloride has a broad spectrum of antimicrobial activity, including activity against gram-positive and gram-negative bacteria, as well as anaerobic bacteria. It is also active against some protozoal infections. In addition to its antimicrobial activity, Minocycline Hydrochloride has been studied for its anti-inflammatory and neuroprotective properties.
Minocycline Hydrochloride is a broad-spectrum antibiotic, tetracycline sensitive or resistant Staphylococcus aureus are effective; the green grape coccus, star actinomycetes, streptococcus pneumoniae and bacteria-like microorganisms, neisseria gonorrhoeae role than other tetracyclines slightly stronger, similar to antimicrobial strength of escherichia coli, proteus, salmonella, shigella, klebsiella pneumoniae, pseudomonas aeruginosa and tetracycline.
1. Minocycline this product is suitable for staphylococcus, streptococcus, pneumococcus, Neisseria gonorrhoeae, dysentery bacillus,Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Treponema pallidum and chlamydia and other sensitive to the product The following infections caused by pathogens: 1. Sepsis, bacteremia.
2. Superficial suppurative infections: folliculitis,pyoderma, tonsillitis, frozen shoulder, dacryocystitis, gingivitis, vulvitis, wound infection, post-operative infection, etc.
3. Deep purulent diseases: mastitis, lymphatic (node) inflammation, submandibular gland inflammation, osteomyelitis, osteitis.
4. Acute and chronic bronchitis, asthmatic bronchitis, bronchiectasis, bronchopneumonia, bacterial pneumonia, atypical pneumonia,and purulent lung disease.
5. Diarrhea, enteritis, infectious food poisoning, cholangitis, cholecystitis.
6. Peritonitis.
7. Pyelonephritis, pyelonephritis, pyelonephritis, urethritis, cystitis, prostatitis, epididymitis, intrauterine infection,gonorrhea.
8. Otitis media, paranasal sinusitis, and submandibular gland inflammation.