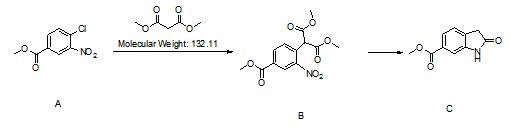
Methyl 2-Oxoindoline-6-Carboxylate CAS 14192-26-8 Purity >99.0% (HPLC) Nintedanib Esylate Intermediate Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Methyl 2-Oxoindoline-6-Carboxylate (CAS: 14192-26-8) with high quality, commercial production.
| Chemical Name | Methyl 2-Oxoindoline-6-Carboxylate |
| Synonyms | 2-Oxoindoline-6-Carboxylic Acid Methyl Ester; Methyl Oxindole-6-Carboxylate |
| CAS Number | 14192-26-8 |
| CAT Number | RF-PI1524 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H9NO3 |
| Molecular Weight | 191.19 |
| Melting Point | 184.0~190.0℃ |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow to Brown Powder |
| 1 H NMR Spectrum | Consistent With Structure |
| Purity / Analysis Method | >99.0% (HPLC) |
| Loss on Drying | <1.00% |
| Total Impurities | <1.00% |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Nintedanib Esylate (CAS: 656247-18-6) |
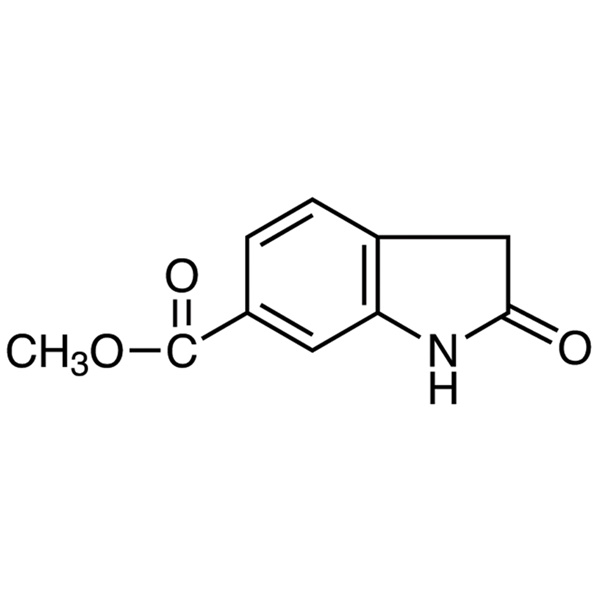
Methyl 2-Oxoindoline-6-Carboxylate (CAS: 14192-26-8) Synthetic Route
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Methyl 2-Oxoindoline-6-Carboxylate (CAS: 14192-26-8) is an intermediate used to prepare Nintedanib Esylate (CAS: 656247-18-6). Nintedanib Esylate is a potent, oral triple angiokinase inhibitor developed by Boehringer Ingelheim that targets proangiogenic and pro-fibrotic pathways mediated by the vascular endothelial growth factor receptor, fibroblast growth factor receptor and plateletderived growth factor receptor families, as well as Src and Flt-3 kinases. Nintedanib Esylate was approved for the treatment of idiopathic pulmonary fibrosis (IPF), a condition in which the lungs become progressively scarred over time, by the US FDA in October 2014 and by the EMA in January 2015. The FDA granted nintedanib esylate fast-track, priority review, orphan product, and breakthrough designations. It was also approved by the EMA in November 2014 for treatment of non-small cell lung cancer in combination with docetaxel after first-line chemotherapy.
-
Methyl 2-Oxoindoline-6-Carboxylate CAS 14192-26...
-
(S)-(-)-Indoline-2-Carboxylic Acid CAS 79815-20...
-
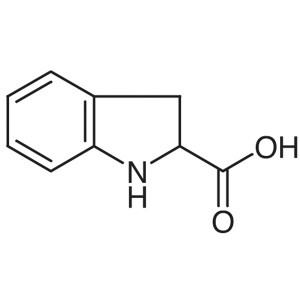
(±)-Indoline-2-Carboxylic Acid CAS 78348-24-0 P...
-
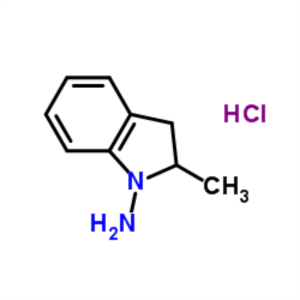
1-Amino-2-Methylindoline Hydrochloride CAS 1027...
-
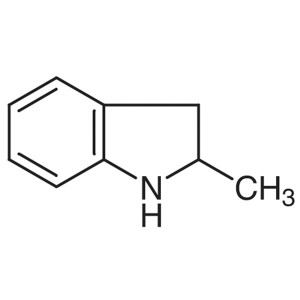
2-Methylindoline CAS 6872-06-6 Purity >99.5% (G...
-
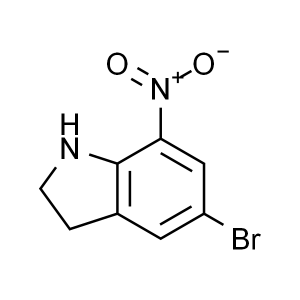
5-Bromo-7-Nitroindoline CAS 80166-90-1 Purity >...
-
Indoline CAS 496-15-1 Purity >99.0% (GC) Factor...
-
1-Boc-Indole CAS 75400-67-8 Purity >96.0% (HPLC)
-
1-Methylindole CAS 603-76-9 Purity >98.0% (GC) ...
-
1-Methylindole-3-Carboxaldehyde CAS 138423-98-0...
-
1-Methylindole-3-Carboxaldehyde CAS 19012-03-4 ...
-
1-Methylindole-3-Carboxylic Acid CAS 32387-21-6...
-
(N-Boc-5-bromo-2-indolyl)boronic Acid CAS 47510...
-
1-(Phenylsulfonyl)indole CAS 40899-71-6 Purity ...
-
1H-Indole-3-Carbohydrazide CAS 15317-58-5 Purit...
-
1H-Indole-7-Carboxamide CAS 1670-89-9 Purity >9...