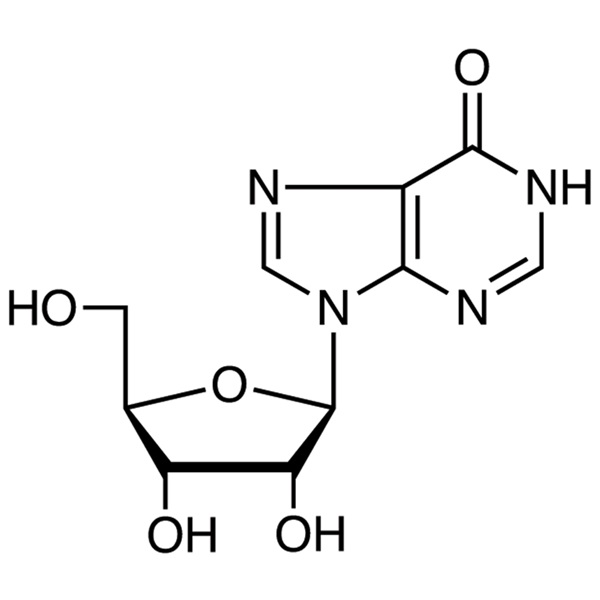
Inosine CAS 58-63-9 Assay 98.0~102.0% Factory High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Inosine (CAS: 58-63-9) with high quality, meets the standard of Chinese Pharmacopoeia ChP2020 II. Our products are mainly sold to America and Europe, India, and the well-known pharmaceutical companies in China. The products have gained high reputation in the international and domestic markets. We can provide worldwide delivery, small and bulk quantities available. If you are interested in Inosine (CAS: 58-63-9), Please contact: alvin@ruifuchem.com
| Chemical Name | Inosine |
| Synonyms | (-)-Inosine; Hypoxanthine 9-β-D-Ribofuranoside; Hypoxanthine-9-beta-D-Ribofuranoside; Atorel; beta-Inosine; Hypoxanthine D-Riboside; 1,9-Dihydro-9-β-D-Ribofuranosyl-6H-purin-6-one; Hypoxanthine Riboside; Hypoxanthine Ribonucleoside; 9-β-D-Ribofuranosylhypoxanthine |
| Stock Status | In Stock, Production Capacity 30 Tons per Month |
| CAS Number | 58-63-9 |
| Molecular Formula | C10H12N4O5 |
| Molecular Weight | 268.23 |
| Melting Point | 222.0~226.0℃(dec.) (lit.) |
| Sensitive | Hygroscopic |
| Solubility | Slightly Soluble in Water, Insoluble in Ethanol, and Soluble in Dilute Hydrochloric Acid and Sodium Hydroxide Solution. |
| Odor | Odorless |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Hazard Codes | Xi - Irritant | RTECS | NM7460000 |
| Risk Statements | 36/37/38 | F | 10 |
| Safety Statements | 24/25-36-26 | TSCA | Yes |
| WGK Germany | 2 | HS Code | 2934993090 |
| Items | Inspection Standards | Results |
| Appearance | White Crystals or Crystalline Powder; Odorless; Slightly Bitter Taste | Conforms |
| Identification | 1) Show Positive Reaction 2) The Retention Time Complies to Standard 3) Infraned Absorption | Conforms |
| State of Solution (Transmittance) | ≥98.0% | Conforms |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Arsenic (As2O3) | ≤1.0ppm | <1.0ppm |
| Loss on Drying | ≤1.00% (at 105℃ for 3 Hours) | 0.40% |
| Residue on Ignition (Sulfated) | ≤0.20% | 0.07% |
| Related Substances | The total impurities surface area max 1.0% of the general peek area | Conforms |
| Assay | 98.0 to 102.0% | 99.4% |
| pH Value | 4.8 to 5.8 (1.0g in 100ml of H2O) | 5.0 |
| Microbiological Tests | ||
| Total Aerobic Limits |
≤800CFU/g | 40CFU/g |
| Total Mold Limits | ≤80 CFU/g | 10CFU/g |
| Yeast | ≤80 CFU/g | 10CFU/g |
| Escherichia Coli | Negative | Negative |
| Conclusion | Meets the Standard of ChP2020 II | |
Inosine (CAS: 58-63-9) ChP2020 Test Method
Inosine - DEFINITION
The content of C10H12N4O5 shall be between 98.0% and 102.0% calculated on the dried product.
Inosine - Trait This product is white crystalline powder; Odorless. This product is slightly soluble in water, insoluble in ethanol, and soluble in dilute hydrochloric acid and sodium hydroxide solution.
Inosine - IDENTIFICATION
1. Take the right amount of 0.01% solution of this product, add equal volume of 3, 5-dihydroxytoluene solution (take 3, 5-dihydroxytoluene and ferric chloride 0.lg, add hydrochloric acid to make 100ml), mix well, after heating in a water bath for about 10 minutes, a green color was observed.
2. In the chromatogram recorded under the content determination item, the retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference solution.
3. The infrared absorption spectrum of this product should be consistent with that of the control (Spectrum set 605).
Inosine - Analysis
Transmittance of Solution
Take 0.5g of this product, add 50ml of water to dissolve, according to UV-visible spectrophotometry (General rule 0401), determine the transmittance at 430nm wavelength, not less than 98.0%. (For injection)
Related Substances
Take this product, add water to dissolve and dilute to make a solution containing 0.5mg per 1ml as a test solution; Take 1ml for precision measurement, put it in a 100ml measuring flask, dilute it with water to the scale, as a control solution. According to the chromatographic conditions under the content determination item, 20 u1 of the test solution and the control solution are respectively injected into the liquid chromatograph, and the chromatogram is recorded to 2 times of the retention time of the main peak. If there are impurity peaks in the chromatogram of the test solution, the sum of each impurity peak area shall not be greater than the main peak area of the control solution (1.0%).
Loss on Drying
Take this product, dry to constant weight at 105°C, weight loss shall not exceed 1.0% (General rule 0831).
Residue on Ignition (Sulfated)
Not more than 0.1% (for injection), or not more than 0.2% (for oral use) (General rule 0841).
Heavy Metals
Take this product 1.0g, inspection according to law (General Principles 0821 second law), containing heavy metals shall not exceed 10 parts per million.
Abnormal Toxicity
Take this product, add sodium chloride injection to dissolve and dilute the solution containing inosine 10mg per 1ml, check according to law (General rule 1141), should comply with the provisions. (For injection)
Inosine - Content determination
Measured by High Performance Liquid Chromatography (General 0512).
Chromatographic conditions and system suitability test silica gel bonded with eighteen alkyl silane was used as the filler; Methanol-water (10:90) was used as the mobile phase; The detection wavelength was 248nm. Take about 10mg of inosine reference, add 1 mol/L hydrochloric acid solution (1ml), heat in water bath at 80 ℃ for 10 minutes, let it cool, add 1ml of 1 mol/L sodium hydroxide solution, add water to 50ml, 20u1 injection liquid chromatograph, adjust the chromatographic system, the separation degree of inosine peak and adjacent impurity peak should meet the requirements, the number of theoretical plate according to the inosine peak calculation is not less than 2000.
Assay
Take an appropriate amount of this product, accurately weigh it, add water to dissolve and quantitatively dilute it to make a solution containing about 20ug per lml, shake it, and use it as a test solution, A 20ul injection liquid chromatograph was accurately measured and the chromatogram was recorded. An appropriate amount of inosine reference was accurately weighed and determined by the same method, and the peak area was calculated according to the external standard method.
Inosine - Category
Cell metabolism improving drugs.
Inosine - Storage
Light shielding, sealed storage.
Pharmaceuticals: (1) Inosine Oral Solution (2) Inosine Tablets (3) Inosine Injection (4) Inosine Capsules (5) Inosine and Glucose Injection (6) Inosine and Sodium Chloride Injection (7) Inosine for Injection
Package: Fluorinated Bottle, 25kg/bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
1. Inosine (CAS: 58-63-9), as a nucleoside product, is mainly used to produce drugs for the treatment of viral infections and other diseases
2. Inosine is a non-canonical nucleotide majorly present as monophosphate. It has ability to base pair with deoxythymidine, deoxyadenosine and deoxyguanosine. Incorporation of inosine in place of guanine modulates translational events. Inosine has antioxidant, anti-inflammatory and neuroprotective functionality. Inosine is prescribed as a therapeutic supplement for nerve injury, inflammation and oxidative stress. It modulates biological processes through adenosine receptors. Its enhances neurite outgrowth in depressive disorders via adenosine receptors. Inosine is also used for treating sepsis in infections.
3. Inosine is the important material of human body, a kind of coenzyme drug widely used in the medicine and the food industry. It can penetrate cell membrane into the cell of body directly, and make the cell in a state of feeblemindedness and anoxia continue to metabolize smoothly. And it can activate caetone acid oxides, and participate the body protein in synthesizing.
Applications of Inosine pharmaceuticals, such as Inosine Tablets and Inosine Injection
1. Used in the treatment of leucopenia and thrombocytopenia.
2. In the treatment of acute hepatitis and chronic hepatitis and liver cirrhosis, hepatic encephalopathy.
3. Used for coronary atherosclerotic heart disease (coronary heart disease), myocardial infarction, rheumatic heart disease, cor pulmonale auxiliary drug use.
4. Used to prevent and mitigate the schistosomiasis prevention drugs caused by the heart and the liver toxicity.
5. Used for eye diseases (central retinal inflammation, optic atrophy) auxiliary drug use.
-
Inosine CAS 58-63-9 Assay 98.0~102.0% Factory H...
-
Adenine CAS 73-24-5 Assay 98.0%~102.0% (Titrati...
-
Adenosine CAS 58-61-7 Assay 99.0%-101.0% USP St...
-
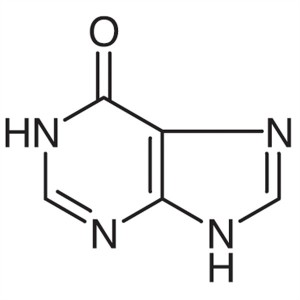
Hypoxanthine CAS 68-94-0 Assay 98.0%~102.0% (UV...
-
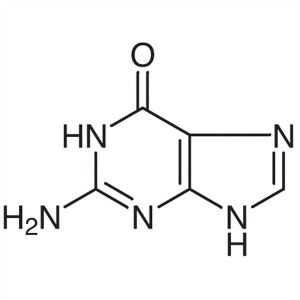
Guanine CAS 73-40-5 Purity ≥99.5% (HPLC) Factor...
-
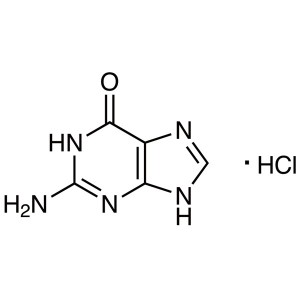
Guanine Hydrochloride CAS 635-39-2 Purity ≥99.5...
-
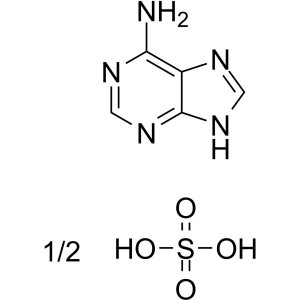
Adenine Hemisulfate Salt CAS 321-30-2 Purity ≥9...
-
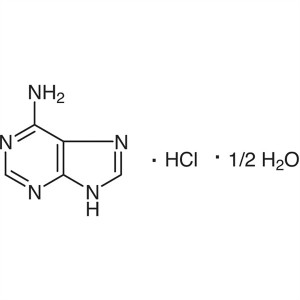
Adenine Hydrochloride Hemihydrate CAS 2922-28-3...
-
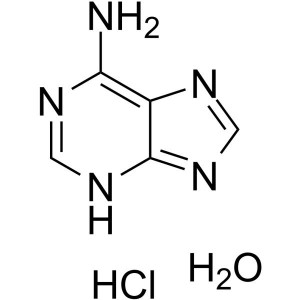
Adenine Hydrochloride Hydrate CAS 6055-72-7 Ass...
-
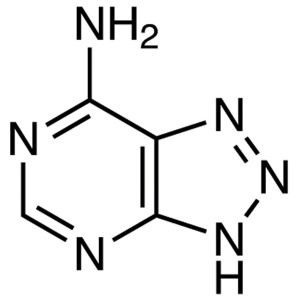
8-Azaadenine CAS 1123-54-2 Assay ≥99.0% (HPLC) ...
-
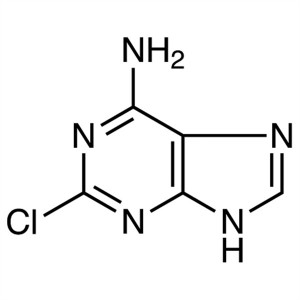
2-Chloroadenine CAS 1839-18-5 Assay ≥98.0% (HPL...
-
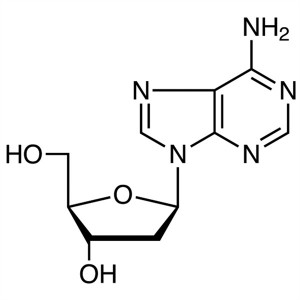
2′-Deoxyadenosine CAS 958-09-8 Purity ≥99...
-
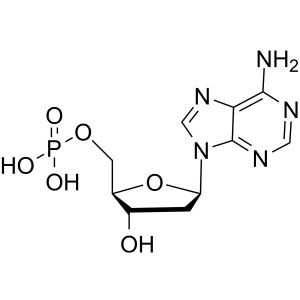
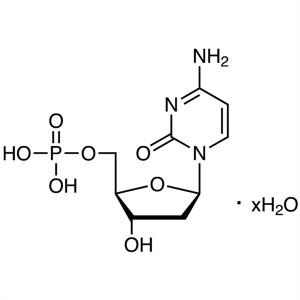
2′-Deoxyadenosine-5′-Monophosphate ...
-
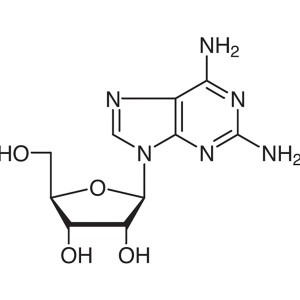
2-Aminoadenosine CAS 2096-10-8 Purity ≥99.0% (H...
-
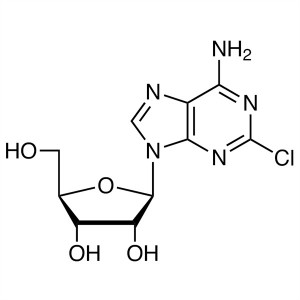
2-Chloroadenosine (2-CADO) CAS 146-77-0 Purity ...
-
2′-Deoxycytidine 5′-Monophosphate H...