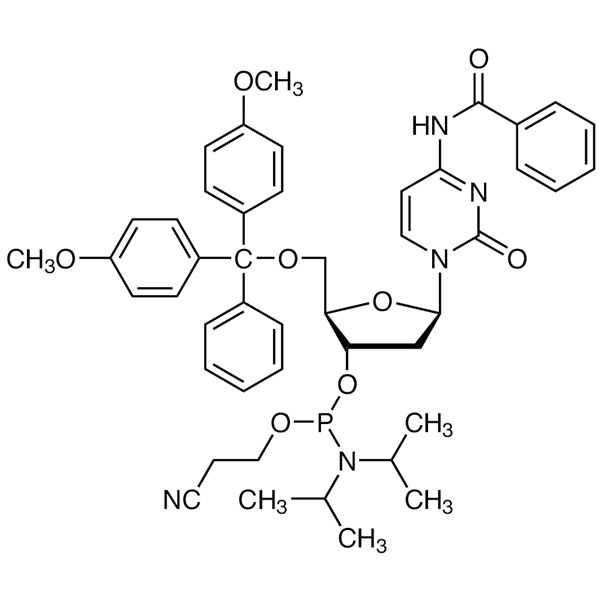
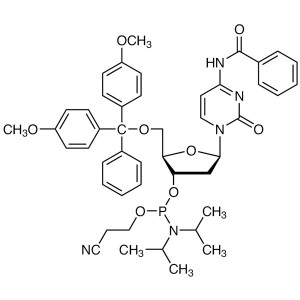
DMT-dC(bz) Phosphoramidite CAS 102212-98-6 Purity ≥98.0% (HPLC) DNA Phosphoramidites
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of DMT-dC(bz) Phosphoramidite (CAS: 102212-98-6) with high quality. DNA Phosphoramidites for Oligonucleotide Synthesis. Used for Molecular Biology Research.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase DMT-dC(bz) Phosphoramidite or other products, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | DMT-dC(bz) Phosphoramidite |
| Synonyms | 5'-O-DMT-N4-Benzoyl-2'-Deoxycytidine 3'-CE Phosphoramidite; dC (N-Bz) Phosphoramidite; dC (Bz) CE-Phosphoramidite; DNAC-Phosphoramidite; N4-Benzoyl-2'-Deoxy-5'-O-DMT-Cytidine 3'-CE Phosphoramidite; N-Benzoyl-5'-O-(4,4'-Dimethoxytrityl)-2'-Deoxycytidine-3'-(2-Cyanoethyl-N,N-Diisopropyl)phosphoramidite; N-Benzoyl-5'-O-[bis(4-Methoxyphenyl)(phenyl)methyl]-3'-O-[(2-Cyanoethoxy)(diisopropylamino)phosphino]-2'-Deoxycytidine |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 102212-98-6 |
| Molecular Formula | C46H52N5O8P |
| Molecular Weight | 833.92 g/mol |
| Melting Point | >84℃(dec.) |
| Density | 1.23 at 20℃ |
| Store Under Inert Gas | Store Under Inert Gas |
| Sensitive | Moisture Sensitive, Heat Sensitive |
| Storage Temperature | Sealed in Dry, Store at -20℃, Protect From Light, Stored Under Nitrogen |
| Shipping Conditions | Shipped Under Ambient Temperature For Short Term |
| Expiration Date | 12 Months From Date of Receipt |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
DNA Phosphoramidites |
| Caution | Not For Human or Veterinary Use. For Research Use Only. |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Off-White Powder | White Powder |
| Water Content | ≤0.30% (Karl Fischer) | Conforms |
| Purity / Analysis Method | ≥98.0% (HPLC) | 99.5% |
| Purity / Analysis Method | ≥98.0% (31P-NMR) | Conforms |
| 1H NMR Spectrum | Conforms to Structure | Conforms |
| LC-MS | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: 1g/10g/50g/100g/1kg/10kg/bulk. Amber glass screw cap vial or white plastic bottle.
Storage Condition: Keep the container tightly closed. Sealed in dry, store in freezer, store at -20℃, protect from light, stored under nitrogen. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| Safety Description | 24/25 - Avoid contact with skin and eyes. |
| WGK Germany | 3 |
| HS Code | 2934.99.9001 |
DMT-dC(bz) Phosphoramidite (CAS: 102212-98-6) is a nucleoside phosphoramidite with a fluorine on the 2' position and a cytidine capped with a benzoyl functional group.
DMT-dC(bz) Phosphoramidite is an important chemical reagent used in the synthesis of oligonucleotides for a variety of scientific applications. It is a derivative of the nucleoside dC and a phosphoramidite, which is a type of phosphite. This compound is used in the synthesis of DNA and RNA molecules and is particularly useful in the construction of oligonucleotide libraries. It is also used in the synthesis of modified oligonucleotides, such as those containing modified bases or modified linkages.
DMT-dC(bz) Phosphoramidite (CAS: 102212-98-6) is used in a variety of scientific research applications. It is used in the synthesis of oligonucleotide libraries, which are used for a variety of purposes such as gene expression analysis, gene function analysis, and drug discovery. DMT-dC(bz) Phosphoramidite is also used in the synthesis of modified oligonucleotides, such as those containing modified bases or modified linkages. These modified oligonucleotides are useful for a variety of applications, such as gene silencing and gene editing.
DMT-dC(bz) Phosphoramidite can be used to study the interaction of DNA with drugs, including anticancer drugs, antibiotics, and antivirals.
DMT-dC(bz) Phosphoramidite can be used in the development of DNA-based sensors for the detection of environmental toxins, pathogens, and other analytes.
The mechanism of action of DMT-dC(bz) Phosphoramidite (CAS: 102212-98-6) is based on the formation of a phosphoramidite linkage between a nucleoside and a phosphite. In the presence of a base and a catalyst, a phosphite reacts with a nucleoside to form a phosphoramidite. The phosphoramidite is then reacted with a protecting group, which is then removed to yield the desired product.
DMT-dC(bz) Phosphoramidite (CAS: 102212-98-6) is a chemical reagent and does not have any direct biochemical or physiological effects. However, it is used in the synthesis of oligonucleotides, which can have a variety of biochemical and physiological effects. For example, oligonucleotides can be used to regulate gene expression, which can lead to changes in cellular physiology.
The main advantage of using DMT-dC(bz) Phosphoramidite in lab experiments is that it is a versatile reagent that can be used in the synthesis of a variety of oligonucleotides. DMT-dC(bz) Phosphoramidite is also relatively easy to use and can be used to synthesize oligonucleotides with modified bases or linkages. The main limitation of using this reagent is that it is relatively expensive, which can limit its use in some experiments.
The use of DMT-dC(bz) Phosphoramidite in the synthesis of oligonucleotides has a number of potential future directions. These include the development of more efficient and cost-effective methods for the synthesis of modified oligonucleotides, the development of new applications for oligonucleotides, such as gene editing and gene silencing, and the development of new methods for the synthesis of oligonucleotides with modified linkages. Additionally, further research into the mechanism of action of this reagent could lead to new applications and improved synthesis methods.
-
DMT-dA(Bz) Phosphoramidite CAS 98796-53-3 Purit...
-
DMT-dC(ac) Phosphoramidite CAS 154110-40-4 Puri...
-
DMT-dC(bz) Phosphoramidite CAS 102212-98-6 Puri...
-
DMT-dG(dmf) Phosphoramidite CAS 330628-04-1 Pur...
-
DMT-dG(Ib) Phosphoramidite CAS 93183-15-4 Purit...
-
DMT-dT Phosphoramidite CAS 98796-51-1 Purity ≥9...
-
I-bu-rG Phosphoramidite CAS 147201-04-5 Purity ...
-
rU Phosphoramidite CAS 118362-03-1 Purity ≥98.0...
-
2′-OMe-A(Bz) Phosphoramidite CAS 110782-3...
-
Ac-rC Phosphoramidite CAS 121058-88-6 Purity ≥9...
-
Bz-rA Phosphoramidite CAS 104992-55-4 Purity ≥9...