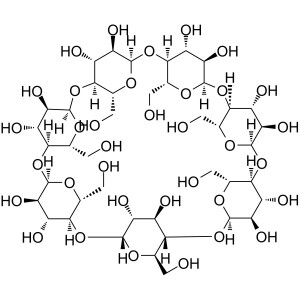
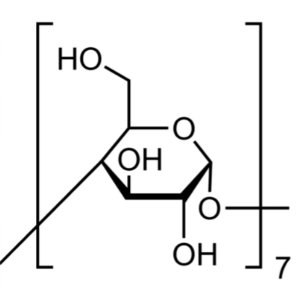
β-Cyclodextrin (β-CD) CAS 7585-39-9 Assay 98.0%~102.0% Factory
Ruifu Chemical is the leading manufacturer of β-Cyclodextrin (β-CD) (CAS: 7585-39-9) with high quality. Ruifu supplies pharma grade cyclodextrins, pharmaceutical excipients. Ruifu can provide worldwide delivery, competitive price, small and bulk quantities available. Purchase β-Cyclodextrin, Please contact: alvin@ruifuchem.com
| Chemical Name | β-Cyclodextrin |
| Synonyms | β-CD; BCD; beta-Cyclodextrin; Cycloheptaamylose; Schardinger β-Dextrin; Caraway; Cyclomaltoheptaose; Betadex |
| Stock Status | In Stock, Production Capacity 3000 Tons per Year |
| CAS Number | 7585-39-9 |
| Molecular Formula | C42H70O35 |
| Molecular Weight | 1,134.99 |
| Melting Point | 290.0~300.0℃(dec.) (lit.) |
| Density | 1.44 g/cm3 at 20℃ |
| Water Solubility | Soluble in Water |
| Solubility in Hot Water | Almost Transparency |
| Stability | Stable. Incompatible with Strong Oxidizing Agents. |
| COA & MSDS | Available |
| Free Sample | Available |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystalline Powder, Odorless, Slightly Sweet Taste | Conforms |
| Identification | Iodine Test: Yellow-Brown Precipitate | Conforms |
| Assay | 98.0%~102.0% | 99.9% |
| Clarity and Color of Solution | Clear and Colorless Solution | Conforms |
| pH of 1% Aqueous Solution | 5.0~8.0 | 6.1 |
| Reducing Sugars | ≤0.20% | <0.20% |
| Light-Absorbing Impurities | ≤0.10 (230nm-350nm) ≤0.05 (230nm-350nm) | Conforms |
| Specific Rotation [a]20/D | +159.0° to +164.0° | +161.5° |
| Loss on Drying | ≤14.0% | 11.5% |
| Residue on Ignition | ≤0.10% | ≤0.05% |
| Heavy Metals | ≤10ppm | <5ppm |
| Chloride | ≤0.018% | <0.018% |
| Total Aerobic Microbial Count | ≤1000cfu/g | Conforms |
| Total Moulds & Yeasts Count | ≤100cfu/g | Conforms |
| Salmonella | Absent/10 g | Conforms |
| E. Coli | Absent/1 g | Conforms |
| Alpha Cyclodextrin | ≤0.25% | None |
| Gamma Cyclodextrin | ≤0.25% | None |
| Other Related Substances | ≤0.50% | None |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the Standard USP35 | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture. Incompatible with oxidizing agents.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Specific rotation
Take this product, weigh it precisely, add water to dissolve and quantitatively dilute it into a solution containing about 10 mg per 1 ml, and determine it according to law (General rule 0621), the specific rotation was 159°to 164°.
Differential diagnosis
Take about 0.2g of this product, add 2ml of Iodine test solution, heat it in a water bath to dissolve it, and let it cool to produce yellow-brown precipitate.
In the chromatogram recorded under the content determination item, the retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the control solution. 6
The infrared absorption spectrum of this product should be consistent with that of the reference product (General rule 0402).
Absorbance of impurities
Take this product about 1 g, precision weighing, add water 100ml to dissolve, according to UV-visible spectrophotometry (General rule 0401) determination, the absorbance in the wavelength range of 230~350nm should not exceed 0.10, the absorbance in the wavelength range of 350~750nm should not exceed 0.05.
pH
take 0.20g of this product, add 20ml of water to dissolve, add 0.2ml of saturated potassium chloride solution, and determine according to law (General rule 0631), pH value should be 5.0~8.0.
Clarity and color of solution
Take this product 0. 50g, add 50ml of water to dissolve, check according to law (General rule 0901 and general rule 0902), the solution should be clear and colorless; In case of turbidity, compare with No. 2 Turbidity standard solution (General rule 0902, first method), not more concentrated.
Chloride
Take this product 0.39G, checked according to law (General rule 0801), and standard sodium chloride solution 7.0ml should not be more concentrated than the control solution (0.018%).
Reducing sugar
Take this product 1.0 g, precision weighing, add water 25ml to dissolve, Add alkaline copper tartrate test solution 40tnl, slowly boil for 3 minutes, place at room temperature overnight, filter with 4# vertical melting funnel, the precipitate was washed with warm water until the washing solution was neutral. The filtrate and washing solution were discarded, while hot with potassium permanganate titration solution (0 .0M o l/L) titration. According to the dry product, the consumption of potassium permanganate titrant (0.02 Mol/l) per L g should not exceed 3.2ml(1.0%).
Cyclohexane
Take about 0.2g of this product, precision weighing, in the top empty bottle, add the internal standard solution (take the right amount of dichloroethylene, add 20% dimethyl sulfoxide solution to make the solution containing about 0.04 in each lm l, (Ready) 10.0ml, as the test solution; Another precision weighing cyclohexane, plus internal standard solution to make about 1 l/l containing cyclohexane 0.078mg of solution, measure 10.0ml in a top empty bottle as a control solution. According to the test of residual solvent determination (General rule 0861), the capillary column with 100% dimethylpolysiloxane as stationary liquid is used as the chromatographic column; The column temperature is 90℃; The inlet temperature is 200℃; The detector temperature is 250℃; the Headspace bottle equilibration temperature was 70℃ and the equilibration time was 20 minutes. Take the reference solution into the headspace, the separation degree between the peaks of each component should be in accordance with the provisions. The test solution and the reference solution are respectively injected in the headspace, the chromatogram is recorded, and the peak area is calculated according to the internal standard method.
Loss on drying
Take this product, dry to constant weight at 105℃, weight loss shall not exceed 14.0% (General rule 0831).
Ignition residue
The 1.0 g of this product shall be taken for inspection according to law (General rule 0841), and the remaining residue shall not exceed.
Heavy metals
The residue left under the item of taking the ignition residue shall not contain more than 10 parts per million of heavy metal when examined by law (General Principles 0821, Law II).
Microbial limit
This product shall be taken and inspected according to law (General Principles 1105 and 1106). The total number of aerobic bacteria per l g of test product shall not exceed 100cfu, the total number of molds and yeasts shall not exceed 100cfu, E. Coli should not be detected.
Content determination
Measured by high performance liquid chromatography (General 0512).
Chromatographic conditions and system suitability test a Eighteen alkyl silane-bonded silica gel was used as a filler; Water-methanol (85 : 15) was used as a mobile phase; And the measurement was performed with a differential refractive index detector. The theoretical plate number is not less than 1500 calculated as the betal cyclodextrin peak.
determination method: take about 50mg of this product, weigh it accurately, put it in a 10ml measuring flask, add an appropriate amount of water to dissolve and dilute to the scale, shake well, and use it as a test solution, 10/xl was injected into the liquid chromatograph accurately, and the chromatogram was recorded. Another 50mg of the reference substance of betaloc cyclodextrin was measured accurately, and the same method was used for determination. According to the external standard method to calculate the peak area, that is.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes
R36/37/38 - Irritating to eyes, respiratory system and skin.
R20 - Harmful by inhalation
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
S24/25 - Avoid contact with skin and eyes.
WGK Germany 2
RTECS GU2293000
TSCA Yes
HS Code 3505100000
β-Cyclodextrin (β-CD) (CAS: 7585-39-9), Cyclodextrins refer to a family of compounds consisting of sugar molecules bound together in ring (cyclic oligosaccharides). It is produced from starch through enzymatic conversion. Beta-cyclodextrin is the 7-membered sugar ring molecular form of cyclodextrin. Cyclodextrin has various applications. In the pharmaceutical industry, it can be used as complexing agents for increasing the solubility of poorly soluble drug as well as increasing their bioavailability and stability. It can also alleviate the gastrointestinal drug irritation, and prevent drug-drug and drug-excipient interactions. It can also be used in food, pharmaceutical, drug delivery, and chemical industries, as well as agriculture and environmental engineering.
β-Cyclodextrin is used as a complexing agent in drug delivery because it forms an inclusion complex with a drug molecule. The complex of cyclodextrin increases the aqueous solubility, dissolution rate and bioavailability of poorly water-soluble drugs, which is useful for the delivery of the medical agent to a biological system.
Cyclodextrins occur as white, practically odorless, fine crystalline powders, having a slightly sweet taste. Some cyclodextrin derivatives occur as amorphous powders. Beta-Cyclodextrin is the most abundant and cheap cyclic oligosaccharide that forms inclusion complexes with several drug molecules. Its main application is in tablet and capsule formulations.
Cyclodextrins are ‘bucketlike’ or ‘conelike’ toroid molecules, with a rigid structure and a central cavity, the size of which varies according to the cyclodextrin type. The internal surface of the cavity is hydrophobic and the outside of the torus is hydrophilic; this is due to the arrangement of hydroxyl groups within the molecule. This arrangement permits the cyclodextrin to accommodate a guest molecule within the cavity, forming an inclusion complex.
Cyclodextrins may be used to form inclusion complexes with a variety of drug molecules, resulting primarily in improvements to dissolution and bioavailability owing to enhanced solubility and improved chemical and physical stability.
Cyclodextrin inclusion complexes have also been used to mask the unpleasant taste of active materials and to convert a liquid substance into a solid material.
β-Cyclodextrin is the most commonly used cyclodextrin, although it is the least soluble. It is the least expensive cyclodextrin; is commercially available from a number of sources; and is able to form inclusion complexes with a number of molecules of pharmaceutical interest. However, β-Cyclodextrin is nephrotoxic and should not be used in parenteral formulations. β-Cyclodextrin is primarily used in tablet and capsule formulations.
In oral tablet formulations, β-Cyclodextrin may be used in both wet-granulation and direct-compression processes. The physical properties of β-Cyclodextrin vary depending on the manufacturer. However, β-Cyclodextrin tends to possess poor flow properties and requiresalubricant,such as 0.1% w/w magnesium stearate,when it is directly compressed.
In parenteral formulations, cyclodextrins have been used to produce stable and soluble preparations of drugs that would otherwise have been formulated using a nonaqueous solvent.
In eye drop formulations, cyclodextrins form water-soluble complexes with lipophilic drugs such as corticosteroids. They have been shown to increase the water solubility of the drug; to enhance drug absorption into the eye; to improve aqueous stability; and to reduce local irritation.
Cyclodextrins have also been used in the formulation of solutions,suppositories, and cosmetics.
Cyclodextrins are starch derivatives and are mainly used in oral and parenteral pharmaceutical formulations. They are also used in topical and ophthalmic formulations.
Cyclodextrins are also used in cosmetics and food products, and are generally regarded as essentially nontoxic and nonirritant materials. However, when administered parenterally, β-Cyclodextrin is not metabolized but accumulates in the kidneys as insoluble cholesterol complexes, resulting in severe nephrotoxicity.
Cyclodextrin administered orally is metabolized by microflora in the colon, forming the metabolites maltodextrin, maltose, and glucose; these are themselves further metabolized before being finally excreted as carbon dioxide and water. Although a study published in 1957 suggested that orally administered cyclodextrins were highly toxic, more recent animal toxicity studies in rats and dogs have shown this not to be the case, and cyclodextrins are now approved for use in food products and orally administered pharmaceuticals in a number of countries.
Cyclodextrins are not irritant to the skin and eyes, or upon inhalation. There is also no evidence to suggest that cyclodextrins are mutagenic or teratogenic.
β-Cyclodextrin
LD50 (mouse, IP): 0.33 g/kg(16)
LD50 (mouse, SC): 0.41 g/kg
LD50 (rat, IP): 0.36 g/kg
LD50 (rat, IV): 1.0 g/kg
LD50 (rat, oral): 18.8 g/kg
LD50 (rat, SC): 3.7 g/kg
Included in the FDA Inactive Ingredients Database: α-Cyclodextrin (injection preparations); β-Cyclodextrin (oral tablets, topical gels); γ-Cyclodextrin (IV injections). Included in the Canadian List of Acceptable Non-medicinal Ingredients (stabilizing agent; solubilizing agent ); and in oral and rectal pharmaceutical formulations licensed in Europe, Japan, and the USA.
Function
1. To increase the solubility of medicine and biological availability
2. To improve the bioavailability of drugs.
3. To adjust or control the releasing of drugs.
4. To decrease the toxicities of drugs.
5. To improve the stabilities of drugs.
Application
Cosmetic:
1. To suit for encapsulation of volatile compounds.
2. To increase shelf life of expensive flavours.
Food:
1. To prevent evaporation of volatile substances.
2. To get rid of the terrible smell.
3. To significantly enhance the effects of emulsification.
4. To make preservative of food release.
1. β-Cyclodextrin is used in the food industry to release food preservatives and preservatives slowly, improve the preservative effect, prolong the shelf life of food, and improve the taste of food, enhance emulsifying ability and moisture resistance.
2. β-Cyclodextrin is widely used in the pharmaceutical excipient industry to increase the stability of the drug, prevent the oxidation and decomposition of the drug, improve the dissolution and bioavailability of the drug, reduce the toxic and side effects of the drug, and mask the odor of the drug and stench.
3. In the cosmetic industry, β-Cyclodextrin can prevent cosmetic whitening agents from damaging blood acid oxidation and browning, improve whitening effect and reduce irritation.
-
β-Cyclodextrin (β-CD) CAS 7585-39-9 Assay 98.0%...
-
α-Cyclodextrin (α-CD) CAS 10016-20-3 Pharmaceut...
-
γ-Cyclodextrin (γ-CD) CAS 17465-86-0 Assay 98.0...
-
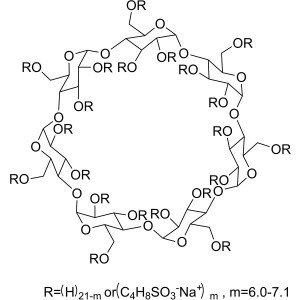
SBE-β-CD CAS 182410-00-0 Betadex Sulfobutyl Eth...
-
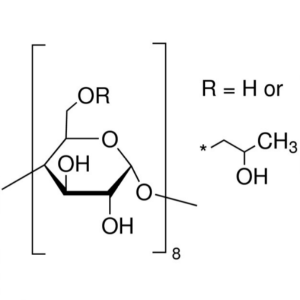
(2-Hydroxypropyl)-γ-Cyclodextrin CAS 128446-34-...
-
Carboxymethyl-β-Cyclodextrin CAS 218269-34-2 (C...
-
Hydroxypropyl-β-Cyclodextrin CAS 128446-35-5 (H...
-
Methyl-β-Cyclodextrin CAS 128446-36-6 (Me-β-CD)