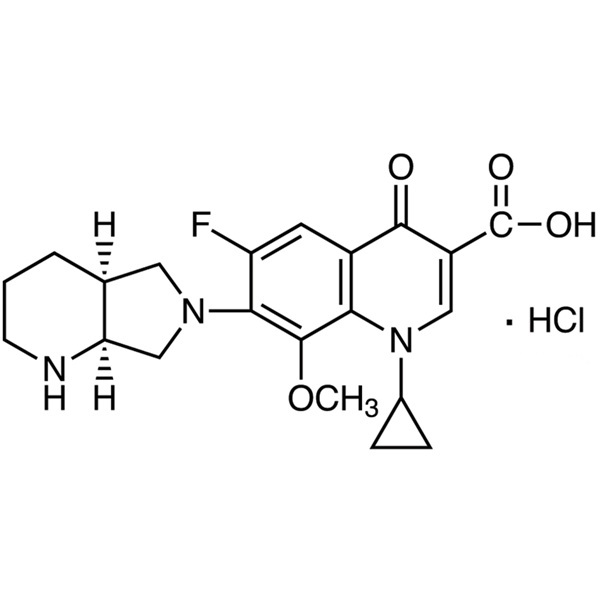
Moxifloxacin Hydrochloride CAS 186826-86-8 Assay 98.0~102.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Moxifloxacin Hydrochloride (CAS: 186826-86-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Moxifloxacin Hydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Moxifloxacin Hydrochloride |
| Synonyms | Moxifloxacin HCl; Moxifloxacin HCl Monohydrate; Moxifloxacin Hydrochloride Monohydrate; BAY 12-8039; 1-Cyclopropyl-6-Fluoro-1,4-Dihydro-8-Methoxy-7-[(4aS,7aS)-Octahydro-1H-Pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-Quinolinecarboxylic Acid Hydrochloride Monohydrate |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 186826-86-8 |
| Related CAS | 192927-63-2 (Moxifloxacin HCl Monohydrate) & 151096-09-2 (Moxifloxacin) |
| Molecular Formula | C21H24FN3O4·HCl |
| Molecular Weight | 437.89 g/mol |
| Melting Point | 231.0~238.0℃ |
| Solubility | Sparingly Soluble in Water. Soluble in DMSO |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Shelf Life | 2 Years When Properly Stored |
| Product Categories | API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | Light Yellow to Yellow Crystalline Powder | Conforms |
| Infrared Spectrum | Conforms to Structure | Conforms |
| Specific Rotation | -125.0° to -138.0° | -129.16° |
| pH | 3.9~4.6 | 4.2 |
| Water by Karl Fischer | <4.50% | 4.09% |
| Residue on Ignition | <0.10% | 0.07% |
| Sulfate | <0.04% | <0.04% |
| Related Compounds | ||
| Moxifloxacin Related compound A | <0.10% | <0.10% |
| 6,8-Dimethoxy Impurity | <0.10% | <0.10% |
| 8-Ethoxy Impurity | <0.10% | <0.10% |
| 6-Methoxy-8-Fluoro Impurity | <0.10% | <0.10% |
| 8-Hydroxy Impurity | <0.10% | <0.10% |
| Other Individual Impurity | <0.10% | <0.10% |
| Total Impurities | <0.50% | <0.50% |
| Microbial Enumeration Tests | ||
| Total Aerobic Microbial Count | <1000 cfu/g | <1000 cfu/g |
| Total Combined Molds and Yeasts | <100 cfu/g | <100 cfu/g |
| Assay / Analysis Method | 98.0~102.0% (HPLC) | 99.73% |
| Conclusion | The product has been tested and complies with the USP35 Standard | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
Moxifloxacin Hydrochloride
C21H24FN3O4·HCl 437.89
(4aS-cis)-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-(octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl)-4-oxo-3-quinolinecarboxylic acid, monohydrochloride.
1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride [186826-86-8].
» Moxifloxacin Hydrochloride contains not less than 98.0 percent and not more than 102.0 percent of C21H24FN3O4·HCl, calculated on the anhydrous basis.
Packaging and storage- Preserve in tight, light-resistant containers. Store at room temperature.
USP Reference standards <11>-
USP Moxifloxacin Hydrochloride RS
USP Moxifloxacin Related Compound A RS
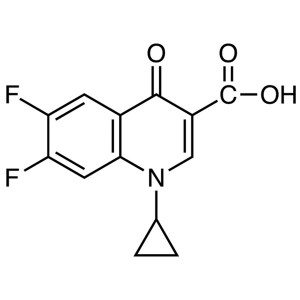
1-Cyclopropyl-6,8-difluoro-1,4-dihydro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid.
C20H21F2N3O3 389.40
Identification-
A: Infrared Absorption <197K>.
B: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
C: To a solution (1 in 160) add diluted nitric acid, and filter. The filtrate meets the requirements of the tests for Chloride 191.
Specific rotation <781S>: between -125° and -138° at 20℃.
Test solution: 10 mg per mL, in a mixture of water and acetonitrile (1:1).
Microbial enumeration tests <61> and Tests for specified microorganisms <62>- The total aerobic microbial count does not exceed 1000 cfu per g, and the total combined molds and yeasts count does not exceed 100 cfu per g.
pH <791>: between 3.9 and 4.6, in a solution (0.2 in 100).
Water, Method Ia <921>: not more than 4.5%.
Residue on Ignition <281>: not more than 0.1%.
Sulfate <221>- A 0.6-g portion shows no more sulfate than corresponds to 0.25 mL of 0.020 N sulfuric acid (0.04%).
Related compounds- [note- Protect solutions from light.]
Mobile phase and Diluent- Prepare as directed in the Assay.
Blank solution- Use the Diluent.
Resolution solution- Prepare as directed in the Assay.
Sensitivity solution- Dilute an accurately measured volume of the Standard solution with Diluent to obtain a solution containing about 0.05 µg per mL. [note-Store the Sensitivity solution under refrigeration and protected from light.]
Standard solution- Dissolve an accurately weighed quantity of USP Moxifloxacin Hydrochloride RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 0.002 mg per mL.
Test solution- Use the Assay preparation.
Chromatographic system (see Chromatography <621>)-Prepare the Chromatographic system as directed in the Assay. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the resolution, R, between moxifloxacin and moxifloxacin related compound A is not less than 1.5. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the column efficiency using the moxifloxacin peak is not less than 4000 theoretical plates; the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%. In addition, chromatograph the Sensitivity solution, and record the peak response as directed for Procedure. Confirm that the signal-to-noise ratio of the moxifloxacin peak is not less than 10.
Procedure- Separately inject equal volumes (about 25 µL) of the Blank solution, the Standard solution, and the Test solution into the chromatograph, record the chromatograms for at least 2 times the retention time of moxifloxacin, and measure the peak responses, disregarding any peaks corresponding to those obtained from the Blank solution. Calculate the percentage of each impurity in the portion of Moxifloxacin Hydrochloride taken by the formula:
(CS / CU)(1/F)(100)(ri / rS)
in which CS is the concentration, in mg per mL, of USP Moxifloxacin Hydrochloride RS in the Standard solution; CU is the concentration, in mg per mL, of Moxifloxacin Hydrochloride in the Test solution; F is the relative response factor for the individual related compound; ri is the peak response of each individual impurity; rS is the peak response of moxifloxacin in the Standard solution; and 100 is the conversion factor to percentage. The limits as shown in Table 1 are met.
Table 1
Related Compound F Relative Retention Time vs. Moxifloxacin Limit (%)
Moxifloxacin related compound A1 1.0 1.15 0.1
6,8-Dimethoxy2 0.71 1.32 0.1
8-Ethoxy3 1.0 1.48 0.1
6-Methoxy-8-fluoro4 1.0 1.71 0.1
8-Hydroxy5 0.29 1.83 0.1
Other individual impurity 1.0 - 0.1
Total impurities - - 0.5
1 1-Cyclopropyl-6,8-difluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
2 1-Cyclopropyl-6,8-dimethoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
3 1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
4 1-Cyclopropyl-8-fluoro-6-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
5 1-Cyclopropyl-6-fluoro-8-hydroxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
Assay-
Buffer solution- Dissolve 0.5 g of tetrabutylammonium hydrogen sulfate and 1.0 g of monobasic potassium phosphate in water, add 2 mL of phosphoric acid, dilute with water to 1000 mL, mix, and pass through a 0.45-µm filter.
Mobile phase- Prepare a degassed mixture of Buffer solution and methanol (18:7). Make adjustments if necessary (see System Suitability under Chromatography 621).
Diluent- Add 20 mg of anhydrous sodium sulfite to 1000 mL of Buffer solution, mix gently, and pass through a 0.45-µm filter.
Resolution solution- Dissolve suitable quantities of USP Moxifloxacin Hydrochloride RS and USP Moxifloxacin Related Compound A RS in Diluent to obtain a solution containing about 0.1 mg per mL and 0.001 mg per mL, respectively.
Standard preparation- Dissolve an accurately weighed quantity of USP Moxifloxacin Hydrochloride RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 0.1 mg per mL.
Assay preparation- Transfer about 50 mg of Moxifloxacin Hydrochloride, accurately weighed, to a 10-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix. Transfer 1.0 mL of this solution to a 50-mL volumetric flask, dilute with Diluent to volume, and mix.
Chromatographic system (see Chromatography <621>)- The liquid chromatograph is equipped with a 293-nm detector and 4.0-mm × 25-cm column that contains 5-µm packing L11. The flow rate is about 0.9 mL per minute. The column temperature is maintained at 45. Chromatograph the Resolution solution as directed for Procedure, and identify the components based on their relative retention times (about 1.0 for moxifloxacin and 1.2 for moxifloxacin related compound A). The resolution, R, between moxifloxacin and moxifloxacin related compound A is not less than 1.5. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the column efficiency using the moxifloxacin peak is not less than 4000 theoretical plates; the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure- Separately inject equal volumes (about 25 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the percentage of C21H24FN3O4·HCl in the portion of Moxifloxacin Hydrochloride taken by the formula:
100(CS / CU)(rU / rS)
in which 100 is the conversion factor to percentage; CS is the concentration, in mg per mL, of USP Moxifloxacin Hydrochloride RS in the Standard preparation; CU is the concentration, in mg per mL, of Moxifloxacin Hydrochloride in the Assay preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| WGK Germany | 2 |
| RTECS | VB1983750 |
| Hazard Class | IRRITANT |
Moxifloxacin Hydrochloride (CAS: 186826-86-8) is a fluoroquinolone antibiotic developed by Bayer Pharmaceuticals (Germany.) It can be used to treat community-acquired pneumonia caused by Staphylococcus aureus, baccilus, pneumococcus, mucositis Moraxella, and Klebsiella pneumoniae, acute chronic bronchitis attacks, and acute sinusitis. For the treatment of adult bacterial lung infections, paranasal sinus, skin, and abdominal cavity. Also used to treat community-acquired pneumonia, chronic bronchitis, urogenital infection, and acute sinusitis. It can be given by mouth, by injection into a vein, and as an eye drop. The fourth generation of ultra broad spectrum quinolones antibiotics. Moxifloxacin Hydrochloride is an antibiotic used to treat adult bacterial infections of the lungs, paranasal sinuses, skin, and abdominal cavity.

![Moxifloxacin Side Chain CAS 151213-42-2 (S,S)-2,8-Diazabicyclo[4,3,0]nonane Purity ≥99.5% (GC) Chiral Purity(HPLC) ≥99.9%](https://www.ruifuchem.com/uploads/Moxifloxacin-Side-Chain-CAS-151213-42-2-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)