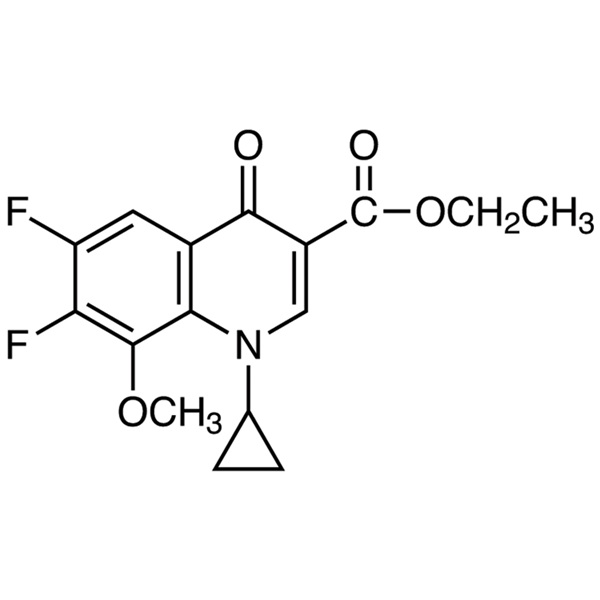
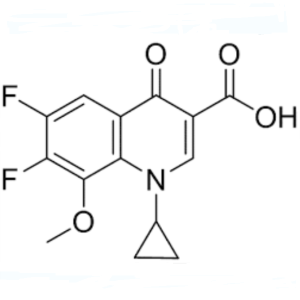
Gatifloxacin Carboxyclic Acid Ethyl Ester CAS 112811-71-9 Purity >99.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Gatifloxacin Carboxyclic Acid Ethyl Ester (CAS: 112811-71-9) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Gatifloxacin and Moxifloxacin Hydrochloride intermediates, Please contact: alvin@ruifuchem.com
| Chemical Name | Gatifloxacin Carboxyclic Acid Ethyl Ester |
| Synonyms | Ethyl 1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-8-Methoxy-4-Oxo-3-Quinolinecarboxylate; Ethyl 1-Cyclopropyl-6,7-Difluoro-8-Methoxy-4-Oxo-1,4-Dihydroquinoline-3-Carboxylate; 1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-8-Methoxy-4-Oxo-3-Quinolinecarboxylic Acid Ethyl Ester; 1-Cyclopropyl-6,7-Difluoro-8-Methoxy-4-Oxo-1,4-Dihydro-Quinoline-3-Carboxylic Acid Ethyl Ester; Gatifloxacin Carboxyclic Acid Ethyl Ester; Moxifloxacin Difluoro Methoxy Ethyl Ester |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 112811-71-9 |
| Molecular Formula | C16H15F2NO4 |
| Molecular Weight | 323.30 g/mol |
| Melting Point | 182.0 to 186.0℃ |
| Density | 1.414±0.06 g/cm3 |
| Sensitive | Heat Sensitive |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results | Test Methods |
| Appearance | Off-White Crystalline Powder | Complies | By Physical Observation |
| Purity / Analysis Method | >99.0% (HPLC) | 99.80% | HPLC |
| Melting Point | 182.0~186.0℃ | 182.5~185.5℃ | CP2005 Version Second Part Addendum VI C |
| Loss on Drying | <0.50% | 0.08% | CP2005 Version Second Part Addendum VIII L |
| Residue on Ignition | <0.20% | 0.08% | CP2005 Version Second Part Addendum VIII N |
| Single Impurity | <0.50% | 0.06% | HPLC |
| Total Impurities | <1.00% | 0.20% | HPLC |
| 1H NMR Spectrum | Consistent with Structure | Complies | |
| Conclusion | The product has been tested and complies with the given specifications | ||
| Application | Intermediate of Gatifloxacin / Moxifloxacin Hydrochloride | ||
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture, avoid fire and heat.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Ethyl 1-Cyclopropyl-6,7-Difluoro-1,4-Dihydro-8-Methoxy-4-Oxo-3-Quinolinecarboxylate (Gatifloxacin Carboxyclic Acid Ethyl Ester) (CAS: 112811-71-9) is an Intermediate of Gatifloxacin (CAS: 112811-59-3) / Moxifloxacin Hydrochloride (CAS: 186826-86-8).
Gatifloxacin is a fourth-generation fluoroquinolone belonging to the racemic group of 8-methofluoroquinolones, with a broad spectrum of anti-gram-negative and positive microorganisms and activity, by the Japanese Xinglin Pharmaceutical Co., Ltd. for the first time successfully developed, the R-and S-enantiomers of the same antibacterial activity, by inhibiting the bacterial DNA gyrase and topoisomerase IV, which inhibit bacterial DNA replication, transcription and repair process to work, has a strong antibacterial effect on a variety of bacteria, it has a good effect on respiratory system infection and reproductive system infection, and is one of the representatives of the "Respiratory fluoroquinolones" drugs that have grown rapidly in recent years. Clinical mainly for the treatment of a variety of mild to moderate infectious diseases caused by sensitive pathogens, including: acute exacerbation of chronic bronchitis, acute sinusitis, Community Acquired Pneumonia, simple urinary tract infection (cystitis) and complex urinary tract infection, cystitis, acute Pyelonephritis and infections of the urethra, cervix, rectum caused by Neisseria gonorrhoeae.
Moxifloxacin Hydrochloride is a fluoroquinolone antibiotic developed by Bayer Pharmaceuticals in Germany. Its mechanism of action and in vitro antibacterial spectrum are similar to other fluoroquinolone drugs, but its antibacterial activity against gram-positive bacteria and anaerobic bacteria is similar to that of travaxacin (trovafloxacin), which is better than some old varieties. Compared with other fluoroquinolones, Gram-positive bacteria resistant to this product are few or very slow. Cross-resistant bacteria with other fluoroquinolones have been found in Gram-negative bacteria and Enterococcus. However, this product is at least effective for staphylococcus aureus isolates grlA,grlB,gyrA or gyrB. This product can inhibit ciprofloxacin-resistant staphylococcus aureus at 0.5~2 mg/L, and is ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin in descending order according to MIC.