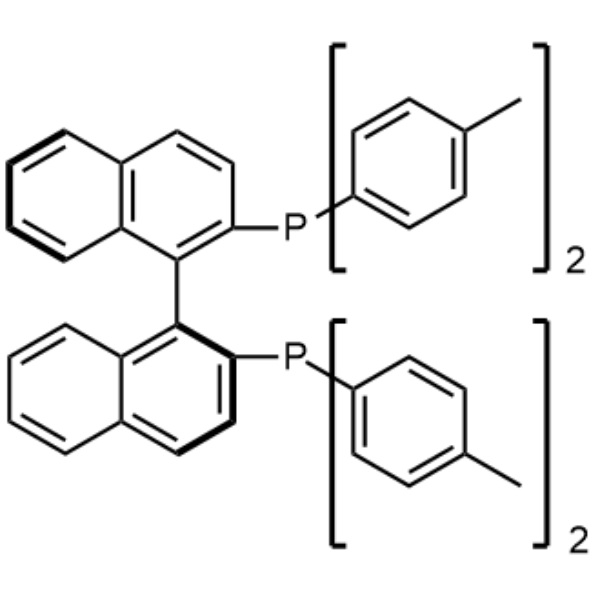
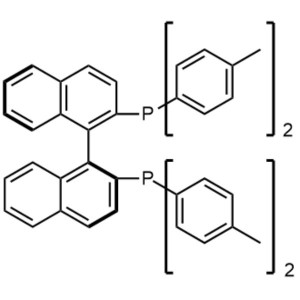
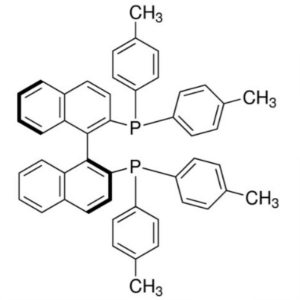
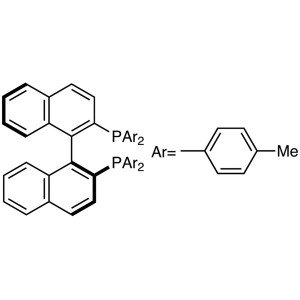
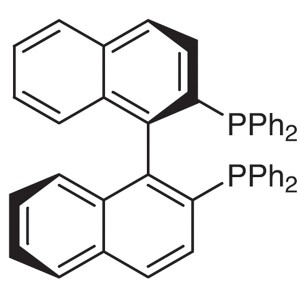
(S)-(-)-TolBINAP CAS 100165-88-6 Purity >98.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of (S)-(-)-TolBINAP (CAS: 100165-88-6) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase (S)-(-)-TolBINAP, Please contact: alvin@ruifuchem.com
| Chemical Name | (S)-(-)-2,2'-Bis(di-p-Tolylphosphino)-1,1'-Binaphthyl |
| Synonyms | (S)-(-)-TolBINAP (S)-Tol-BINAP; (S)-T-BINAP; (S)-Tol-BINA; (-)-Tol-BINA; (-)-Tol-BINAP |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 100165-88-6 |
| Molecular Formula | C48H40P2 |
| Molecular Weight | 678.80 g/mol |
| Melting Point | 252.0~259.0℃ |
| Sensitive | Air Sensitive. Store Under Inert Gas |
| Water Solubility | Insoluble in Water |
| COA & MSDS | Available |
| Product Categories | Ligands; Chiral Ligands |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to Off-White Powder | White Powder |
| Melting Point | 252.0~259.0℃ | 256.9℃ |
| Purity / Analysis Method | >98.0% (HPLC) | 98.5% |
| Purity / Analysis Method | 97.5~102.5% (Neutralization Titration) | Complies |
| Optical Purity (e.e) | >99.0% (HPLC) | 99.2% |
| Specific Rotation [a]20/D | -167.0°~-157.0° (C=1.052, Benzene) | -158.7° |
| Infrared Spectrometry | Consistent with Structure | Complies |
| 1H NMR Spectrum | Consistent with Structure | Complies |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany 3
TSCA No
HS Code 2931900090
(S)-(-)-2,2'-Bis(di-p-Tolylphosphino)-1,1'-Binaphthyl ((S)-(-)-TolBINAP) (CAS: 100165-88-6) Reaction
Useful ligand for palladium-catalyzed carbon-oxygen bond formation.
Ligand for palladium-catalyzed α-arylation of ketones.
Ligand for Cu-catalyzed asymmetric conjugate reduction.
Ligand for Cu-catalyzed asymmetric dienolate addition to aldehydes.
Enantioselective conjugate reduction of lactones and lactams.
Ligand used in the enantioselective cycloaddition of allenylsilanes with α-Imino esters.
Catalytic Aldol reaction to ketones.
Ligand with rhodium catalyses [2+2+2] cycloaddition reaction of alkenes and alkynes.
Ligand used in the iridium-catalyzed enantioselective C-H bond activation of 2-(alkylamino)-pyridine with alkenes.
Iridium-catalyzed regio-, diastereo-, and enantioselective tert-(hydroxyl)-prenylation of alcohols.
Rhodium-catalyzed cross cyclotrimerization.
(S)-(-)-2,2'-Bis(di-p-Tolylphosphino)-1,1'-Binaphthyl ((S)-(-)-TolBINAP) (CAS: 100165-88-6) is chiral ligand widely used in asymmetric synthesis. It also used as excellent catalysts for asymmetric hydrogenation of alkenes and some cyclic anhydrides. BINAP is used in organic synthesis for enantioselective transformations catalyzed by its complexes of ruthenium, rhodium, and palladium.
(S)-(-)-TolBINAP reacts with silver nitrate to form (S)-Tol-BINAP·AgNO3, which can catalyze the enantioselective allylation reaction of aldehydes to form enantiopure secondary alcohols. It may be used as a chiral ligand in the palladium catalyzed asymmetric double carbohydroamination of iodoarenes to form α-aminoamides. It can also catalyze the asymmetric N-allylation reaction of ortho-tert-butylanilide derivatives with diallyl carbonate to form chiral N-allyl ortho-tert-butylanilides.