6-Aminohexanoic Acid CAS 60-32-2 (ε-Aminocaproic Acid) Assay 98.5~100.5% Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of 6-Aminohexanoic Acid (ε-Aminocaproic Acid) (CAS: 60-32-2) with high quality. We can provide worldwide delivery, small and bulk quantities available. If you are interested in 6-Aminohexanoic Acid, Please contact: alvin@ruifuchem.com
| Chemical Name | 6-Aminohexanoic Acid |
| Synonyms | ε-Aminocaproic Acid; ε-Acp; 6-Aminocaproic Acid; (6-)ε-Aminocaproic Acid; Aminocaproic Acid; epsilon-Aminocaproic Acid; EACA; ACS; H-6-Aca-OH; Hemocaprol; 6-Amino-n-Hexanoic Acid; ε-Amino-n-Hexanoic Acid; Amicar |
| Stock Status | In Stock, Production Capacity 500 Tons per Year |
| CAS Number | 60-32-2 |
| Molecular Formula | C6H13NO2 |
| Molecular Weight | 131.18 |
| Melting Point | About 204℃ With Decomposition |
| Density | 1.042 g/cm3 |
| Sensitive | Air Sensitive |
| Odor | Odorless |
| Water Solubility | Freely Soluble in Water, Almost Transparency |
| Solubility | Freely Soluble in Water and in Glacial Acetic Acid, Very Slightly Soluble in Methanol, Practically Insoluble in Chloroform, Ethanol, Ether |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Hazard Codes | Xi - Irritant |
| Risk Statements | 36/37/38 - Irritating to eyes, respiratory system and skin. |
| Safety Description | S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S36 - Wear suitable protective clothing. |
| WGK Germany | 2 |
| RTECS | MO6300000 |
| TSCA | Yes |
| HS Code | 2922491990 |
| Toxicity | LD50 in rats (g/kg): 7.0 i.p.; ~3.3 i.v. (Hallesy) |
| Items | Inspection Standards | Results |
| Appearance | White Crystals or Crystalline Powder; Slightly Bitter Taste | Conforms |
| Identification | Infrared Absorption Spectrum | Conforms |
| State of Solution (Transmittance) | Clear and Colorless ≥98.0% | 98.6% |
| Chloride (Cl) | ≤0.020% | <0.020% |
| Sulfate (SO4) | ≤0.020% | <0.020% |
| Ammonium (NH4) | ≤0.020% | <0.020% |
| Iron (Fe) | ≤30ppm | <30ppm |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Arsenic (As2O3) | ≤1.0ppm | <1.0ppm |
| UV Absorbance | A a1≤0.10 (287nm) a2≤0.03 (450nm) |
a1:0.030 a2:0.006 |
| B a1≤0.15 (287nm) a2≤0.03 (450nm) |
a1:0.121 a2:0.012 | |
| Other Amino Acids | Chromatographically Not Detectable | Conforms |
| Water (by Karl Fischer) | ≤0.50% | 0.20% |
| Residue on Ignition (Sulfated) | ≤0.10% | 0.05% |
| Assay | 98.5 to 100.5% (on the Anhydrous Basis) | 99.8% |
| Ninhydrin-Positive Substances | ≤0.50% | Conforms |
| pH Value | 7.0 to 8.0 (1.0g in 10ml of H2O) | 7.76 |
| Conclusion | Meets with the Standard of AJI97, USP35, EP8.0, BP2005 | |
| Main Uses | Anti-Fibrinolytic Agent; Hemostatic Agent | |
6-Aminohexanoic Acid (ε-Aminocaproic Acid) (CAS: 60-32-2) AJI97 Test Method
ε-Aminocaproic Acid, when calculated on the anhydrous basis, contains not less than 98.5 percent and not more than 100.5 percent of ε-Aminocaproic Acid (C6H13NO2).
Description: White crystals or crystalline powder, slightly bitter taste.
Freely soluble in water and in glacial acetic acid, slightly soluble in methanol, practically insoluble in ethanol.
Solubility (H2O, g/100g): Freely soluble in water
Identification: Compare the infrared absorption spectrum of the sample with that of the standard by potassium bromide disc method.
Specifications:
State of Solution (Transmittance): 0.5g in 10ml of H2O, spectrophotometer, 430nm, 10mm cell thickness.
Chloride (Cl): 0.7g, A-1, ref: 0.40ml of 0.01mol/L HCl
Ammonium (NH4): B-1
Sulfate (SO4): 1.2g, (1), ref: 0.50ml of 0.005mol/L H2SO4
Iron (Fe): 0.5g, ref: 1.5ml of Iron Std. (0.01mg/ml)
Heavy Metals (Pb): 2.0g, (1), pH=7, ref: 2.0ml of Pb Std. (0.01mg/ml)
Arsenic (As2O3): 2.0g, (1), ref: 2.0ml of As2O3 Std.
Other Amino Acids: Test sample: 100μg, B-1-a, control; ε-Acp 0.6μg
Water: 500mg, methanol: ethyleneglycol (1:2) for Karl Fischer Method, A, for 15 minutes.
Residue on Ignition (Sulfated): AJI Test 13
Assay: Sample calculated on the anhydrous basis, 130mg, (1), 3ml of formic acid, 50ml of glacial acetic acid, 0.1mol/L HCLO4 1ml=13.117mg C6H13NO2
pH: 1.0g in 10ml of H2O
Recommended storage limit and condition: Preseved tight containers at controlled room temperature (2 years).
6-Aminohexanoic Acid (ε-Aminocaproic Acid) (CAS: 60-32-2) USP35 Test Method
Aminocaproic Acid contains not less than 98.5 percent and not more than 101.5 percent of C6H13NO2, calculated on the anhydrous basis.
Packaging and storage-Preserve in tight containers. Store at room temperature.
USP Reference standards <11>-
USP Aminocaproic Acid RS
Identification, Infrared Absorption <197K>.
Water, Method I <921>: not more than 0.5%.
Residue on ignition <281>: not more than 0.1%.
Heavy metals, Method II <231>: 0.002%.
Assay-
Solution A-Transfer 0.55 g of sodium 1-heptanesulfonate to a 1000-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Mobile phase-Transfer 10 g of monobasic potassium phosphate to a 1000-mL beaker, dissolve in 300 mL of Solution A, add 250 mL of methanol, followed by another 300 mL of Solution A, and mix. Adjust the mixture with phosphoric acid to a pH of 2.2. Transfer the whole mixture to a 1000-mL volumetric flask, dilute with Solution A to volume, and mix. Filter and degas. Make adjustments if necessary (see System Suitability under Chromatography <621>).
Internal standard solution-Prepare a solution of methionine in water containing 1.25 mg per mL.
Standard preparation-Dissolve an accurately weighed quantity of USP Aminocaproic Acid RS in water to obtain a Stock solution having a known concentration of 12.5 mg per mL. Transfer 5.0 mL of the Stock solution to a 100-mL volumetric flask, add 2.0 mL of the Internal standard solution, dilute with water to volume, and mix.
Assay preparation-Transfer an accurately weighed quantity of 1.25 g of Aminocaproic Acid to a 100-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 100-mL volumetric flask, add 2.0mL of Internal standard solution, dilute with water to volume, and mix.
Chromatographic system (see Chromatography <621>)-The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm × 15-cm column that contains packing L1 and is maintained at 30°. The flow rate is about 0.7 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.76 for aminocaproic acid and 1.0 for methionine; the resolution, R, between aminocaproic acid and methionine is not less than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure-Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, and allow the Assay preparation to elute for not less than two times the retention time of aminocaproic acid. Record the chromatograms, and measure all the peak responses. Calculate the quantity, in g, of C6H13NO2 in the portion of Aminocaproic Acid taken by the formula:
2C(RU / RS)
in which C is the concentration, in mg per mL, of USP Aminocaproic Acid RS in the Standard preparation; and RU and RS are the ratios of the aminocaproic acid peak response to the internal standard peak response obtained from the Assay preparation and the Standard preparation, respectively.
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool, dry and ventilated warehouse away from incompatible substances. Protect from light and moisture. Incompatible with strong oxidizing agents.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
6-Aminohexanoic Acid (ε-Aminocaproic Acid; 6-Aminocaproic Acid) (CAS: 60-32-2) (Brand name: Amicar) is a kind of synthetic derivative of lysine. Since it is a analogue of amino acid lysine, it can act as the inhibitor for enzymes that need to bind to that particular lysine residue, e.g. the proteolytic enzyme such as plasmin, which is responsible for fibrinolysis. Therefore, It has anti-fibrinolytic activity. It also competitively inhibits activation of plasminogen, thereby reducing conversion of plasminogen to plasmin. Based on this property, it can be used for the treatment of acute bleeding due to elevated fibrinolytic activity in many clinical situations. It can also indicated by FDA for the prevention of recurrent hemorrhage in patients of traumatic hyphema. It may also act as a prophylactic against the vascular disease due to its inhibitory effect on the formation of lipoprotein which is the risk factor of vascular disease. Aminobenzoic Acid Gel, Aminocaproic Acid Injection, Aminocaproic Acid Oral Solution, Aminobenzoic Acid Topical Solution.
Application
6-Aminohexanoic Acid was used as a biochemical reagent. 6-Aminocaproic Acid is used in organic synthesis. As an anti-fibrinolytic agent. Used as a hemostatic agent. 6-aminocaproic acid has a significant effect on some severe bleeding caused by increased fibrinolytic activity. It is suitable for oozing or local bleeding during various surgical operations. 6-aminocaproic acid is also used for hemoptysis, gastrointestinal bleeding and bleeding disorders in obstetrics and gynecology. 6-Aminocaproic Acid works by inhibiting the fibrinolytic system. Mainly used for hemorrhage caused by elevated plasmin activity, such as obstetrics and gynecology hemorrhage, hemorrhage after prostate, liver, pancreas, lung and other visceral operations. Early intraoperative medication or preoperative medication can reduce intraoperative oozing and reduce blood transfusion volume.
6-Aminocaproic Acid is an anti-fibrinolytic drug with a similar chemical structure to lysine. It can qualitatively inhibit the binding of plasminogen to fibrin and prevent its activation, thereby inhibiting fibrinolysis and achieving hemostasis. Aminocaproic acid is a monoaminocarboxylic acid, which can inhibit the conversion of plasminogen into plasmin and its binding to fibrin. For severe bleeding caused by hyperfibrinolysis caused by increased activation of plasminogen, can have therapeutic effect.
-

6-Aminohexanoic Acid CAS 60-32-2 (ε-Aminocapro...
-
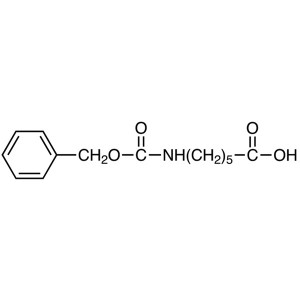
Z-6-Aminohexanoic Acid CAS 1947-00-8 (Z-ε-Acp-O...
-
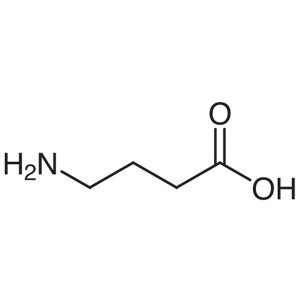
γ-Aminobutyric Acid (GABA) CAS 56-12-2 Assay 99...
-
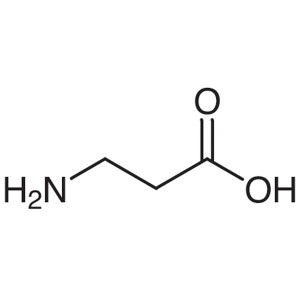
β-Alanine CAS 107-95-9 (H-β-Ala-OH) Assay 98.0~...
-
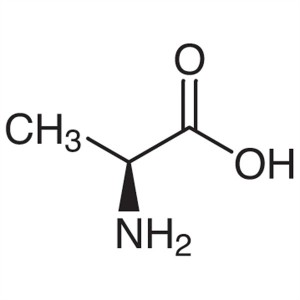
L-Alanine CAS 56-41-7 (H-Ala-OH) Purity 98.5%~1...
-
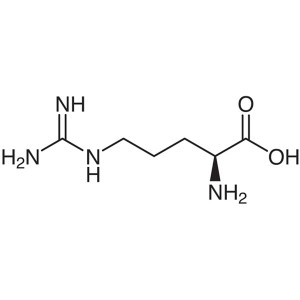
L-Arginine CAS 74-79-3 (H-Arg-OH) Assay 98.5~10...
-
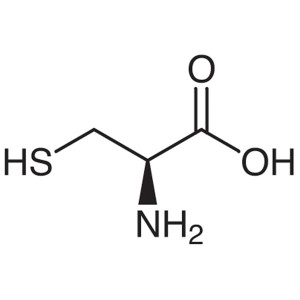
L-Cysteine CAS 52-90-4 (H-Cys-OH) Assay 98.5~10...
-
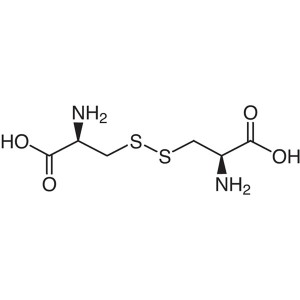
L-Cystine CAS 56-89-3 (H-Cys-OH)2 Assay 98.5~10...
-
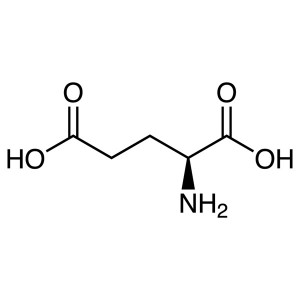
L-Glutamic Acid CAS 56-86-0 (H-Glu-OH) Assay 98...
-
L-Glutamine CAS 56-85-9 (H-Gln-OH) Assay 99.0~1...
-
Glycine CAS 56-40-6 (H-Gly-OH) Assay 98.5~101.5...
-
L-Histidine CAS 71-00-1 (H-His-OH) Assay 98.5~1...
-
L-Isoleucine CAS 73-32-5 (H-Ile-OH) Assay 98.5~...
-
L-Leucine CAS 61-90-5 (H-Ile-OH) Assay 98.5~101...
-
L-(+)-Lysine CAS 56-87-1 (H-Lys-OH) Assay 98.5~...
-
L-Proline CAS 147-85-3 (H-Pro-OH) Assay 98.5~10...


