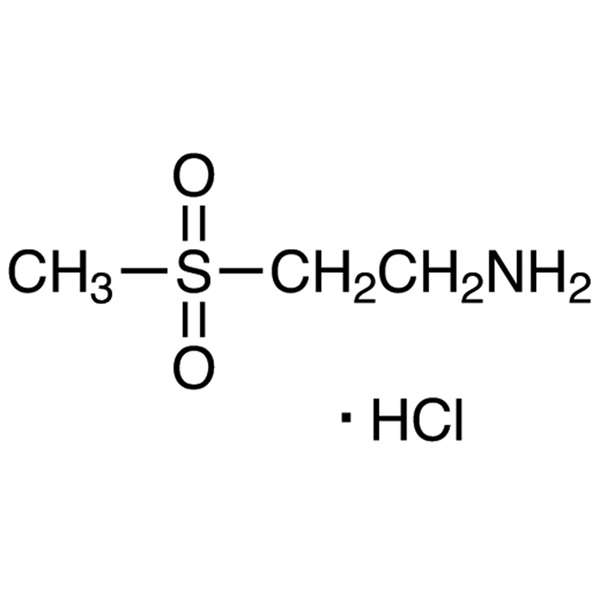
2-Aminoethyl Methyl Sulfone Hydrochloride CAS 104458-24-4 Purity >99.0% (HPLC)
| Chemical Name | 2-Aminoethyl Methyl Sulfone Hydrochloride |
| Synonyms | 2-Aminoethylmethylsulfone Hydrochloride; 2-(Methylsulfonyl)ethanamine Hydrochloride; 2-(Methylsulfonyl)Ethylamine HCl; 2-Aminoethylmethyl Sulfone HCl; Lapatinib Intermediate 1 |
| CAS Number | 104458-24-4 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C3H9NO2S·HCl |
| Molecular Weight | 159.63 |
| Melting Point | 165.0 to 172.0℃ |
| Stability | Hygroscopic |
| Solubility | Soluble in Methanol |
| Sample | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Powder |
| Purity / Analysis Method | >99.0% (HPLC) |
| Melting Point | 165.0~172.0℃ |
| Loss on Drying | <1.00% |
| Residue on Ignition | <0.50% |
| Total Impurities | <1.00% |
| Heavy Metals (as Pb) | <20ppm |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of API (CAS 388082-77-7) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture

2-Aminoethyl Methyl Sulfone Hydrochloride (CAS: 104458-24-4) is an intermediate of API (CAS: 388082-77-7). (CAS: 388082-77-7) is a new drug targeted for the treatment of breast cancer developed by GlaxoSmithKline, which was approved for marketing by the US Food and Drug Administration on March 13, 2007. The currently approved indications are advanced mastocarcinoma treated with (CAS: 388082-77-7) and Capecitabine or metastatic breast cancer, and breast cancer patients must be treated first in other first-class drugs. Its trade name is Tykerb in the United States. On December 14, 2007, the European Medicines Agency (EMEA) approved for marketing in Europe, and the trade name is Tyverb. Breast cancer molecular targeted therapy is the treatment for oncogene related with occurrence and development of breast cancer, and for its related expression product. Molecular targeted drugs control the changes of gene expression in the cell through blocking signal transduction of tumor cell or related cell, thereby inhibiting or killing tumor cells.
-
Lapatinib Base CAS 231277-92-2 Purity ≥99.0% (H...
-
Lapatinib Ditosylate Monohydrate CAS 388082-78-...
-
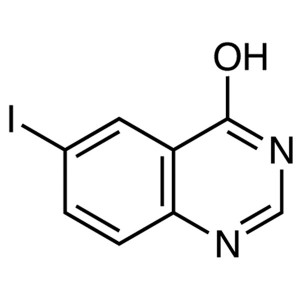
6-Iodo-4-Hydroxyquinazoline CAS 16064-08-7 Puri...
-
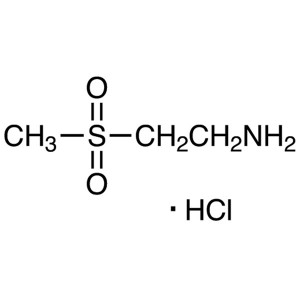
2-Aminoethyl Methyl Sulfone Hydrochloride CAS 1...
-
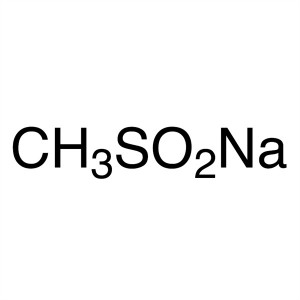
Sodium Methanesulfinate CAS 20277-69-4 Purity >...
-
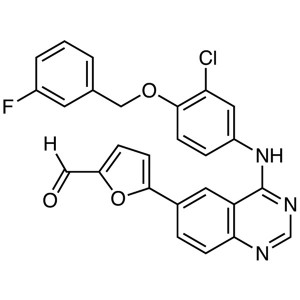
Lapatinib Intermediate CAS 231278-84-5 Purity >...
-
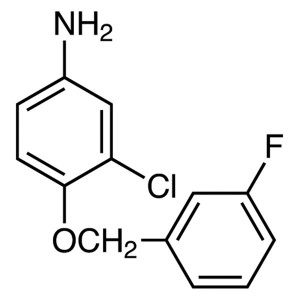
3-Chloro-4-(3-Fluorobenzyloxy)aniline CAS 20219...
-
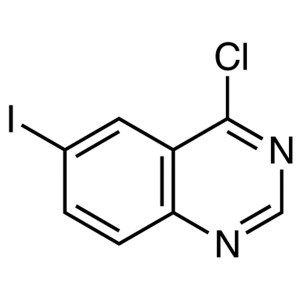
4-Chloro-6-Iodoquinazoline CAS 98556-31-1 Purit...
-
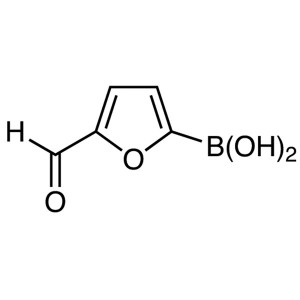
5-Formyl-2-Furanboronic Acid CAS 27329-70-0 Pur...