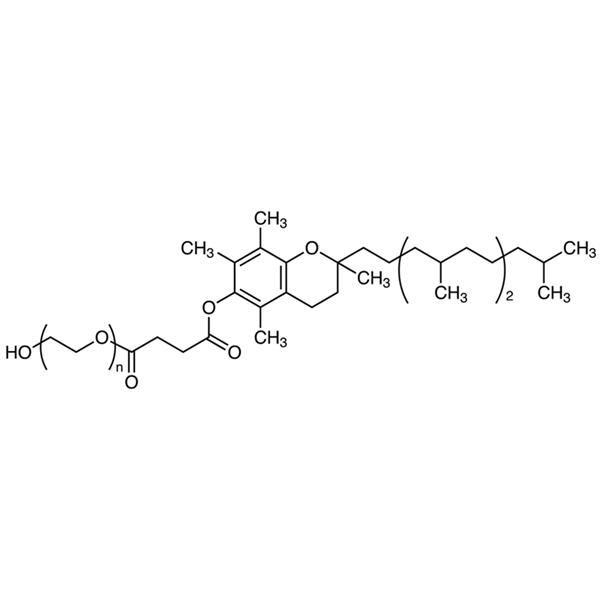
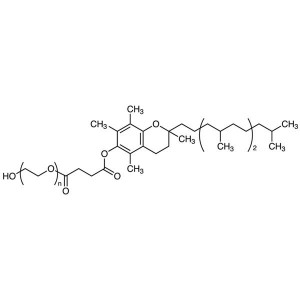
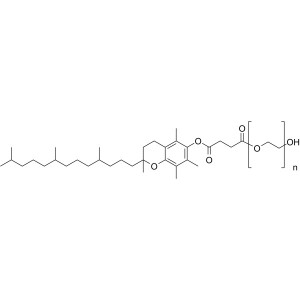
Vitamin E-TPGS (Tocofersolan) CAS 9002-96-4 D-α-Tocopherol ≥25.0% High Quality
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Vitamin E-TPGS (Tocofersolan) (CAS: 9002-96-4) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Vitamin E-TPGS, Please contact: alvin@ruifuchem.com
| Chemical Name | Vitamin E Polyethylene Glycol Succinate |
| Synonyms | Vitamin E-TPGS; TPGS; Tocofersolan; α-Tocopherol Polyethylene Glycol Succinate; D-α-Tocopherol Polyethylene Glycol 1000 Succinate; alpha-Tocopherol Polyethylene Glycol Succinate |
| Stock Status | In Stock, Commercial Scale |
| CAS Number | 9002-96-4 |
| Molecular Formula | C33H54O5.(C2H4O)n |
| Melting Point | 34.0~38.0℃ |
| Density | 1.01g/cm3 |
| Sensitive | Air Sensitive, Light Sensitive, Moisture Sensitive |
| Storage Temp. | Store Long-Term at Cool & Dry Place (2~8℃) |
| Shelf Life | 24 Months When Properly Stored |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Chemical Family | Vitamin E Derivative |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | Off-White to Pale Yellow Waxy Solid |
Complies |
| Identification (GC) | The retention time of the major peak of the sample solution corresponds to that of the Standard solution | Complies |
| Melting Point | 34.0~38.0℃ | 36.0~36.6℃ |
| Assay D-alpha Tocopherol | ≥25.0% (C29H50O2) | 26.4% |
| Free Tocopherol | ≤1.5% | Complies |
| Specific Rotation | ≥+24.0° | +24.5° |
| Solubility in Water | 20g Sample Can be Dissolved in 80ml Boiling Water | Clear Within 3 h |
| Acid Value | ≤0.27ml (0.10 N Sodium Hydroxide) | 0.22ml |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Arsenic (As) | ≤1ppm | <1ppm |
| Lead | ≤3ppm | <3ppm |
| Mercury (Hg) | ≤0.1ppm | <0.010ppm |
| Residual Solvents | ||
| Ethyl Acetate | ≤50ppm | Not Detected |
| Ethanol | ≤50ppm | 23ppm |
| Total Plate Count | ≤1000CFU/g | <10CFU/g |
| Yeasts and Moulds | ≤100CFU/g | <10CFU/g |
| Salmonella | Negative | Negative |
| Escherichia Coli | Negative | Negative |
| Psendomonas Aeruginosa | Negative | Negative |
| Staphylococcus Aureus | Negative | Negative |
| Infrared Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/food-grade cardboard drum, or according to customer's requirement.
Storage Condition: Store in a tightly closed container. Store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
DEFINITION
Vitamin E Polyethylene Glycol Succinate is a mixture formed by the esterification of d-alpha tocopheryl acid succinate and polyethylene glycol. The ester mixture consists primarily of mono-esterified polyethylene glycol and a small amount of di-esterified polyethylene glycol. It contains NLT 25.0% of d-alpha tocopherol (C29H50O2).
IDENTIFICATION
• A. Gas Chromatographic Identification Test
Analysis: Proceed as directed in the test for Content of Alpha Tocopherol.
Acceptance criteria: The retention time of the major peak of the Sample solution corresponds to that of the Standard solution.
COMPOSITION
• Content of Alpha Tocopherol
Solvent: 0.25 mL of phenolphthalein TS in 1 L of alcohol
Internal standard solution: 12 mg/mL of ethyl arachidate in isooctane
Standard solution: Transfer 32.5 mg of USP Alpha Tocopherol RS to a suitable reaction flask. Add 2 mL of pyridine and 0.5 mL of N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane, and heat the flask at 100 for 10 min. Cool the flask, add 5.0 mL of Internal standard solution followed by 20 mL of isooctane, and shake.
Sample solution: Transfer a quantity equivalent to 0.100–0.160 g of Vitamin E Polyethylene Glycol Succinate molten at 60 to a culture tube (about 20 cm long and 2.5 cm in diameter) equipped with a screw cap. Add 40–50 mg of ascorbic acid and a few boiling chips, followed by 20 mL of Solvent. [Note-Reflux the solution gently without emission of contents.] Place the tube in a heating block set at 100–150. When the sample is fully dissolved, add 0.25 g of potassium hydroxide, and continue to reflux for 30 min. Remove the tube from heat, and while contents are still hot, add 1–2 mL of hydrochloric acid dropwise until the pink coloration disappears. [Caution-Exothermic reaction. Allow the acid to trickle down the inside of the tube to prevent splashing.] Cool the tube, then wash the sides of the tube with 20 mL of water. Add 5.0 mL of Internal standard solution, cap, and shake to ensure thorough mixing. Allow the tube to stand until two distinct layers are formed. Transfer 2.5–3.5 mL of the upper layer into a suitable reaction flask, and add 2.0 mL of pyridine followed by 2.5 mL of N,O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane. Heat the flask at 100 for 10 min. Cool, and then add 12 mL of isooctane.
Chromatographic system
(See Chromatography 621, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.25-mm × 15-m fused-silica capillary; coated with a 0.25-µm film of phase G27
Temperature
Injector: 280
Detector: 345
Column: See Table 1.
Table 1
Initial Temperature(°) Temperature Ramp (°/min) Final Temperature (°) Hold Time at Final Temperature (min)
260 20 340 1
Carrier gas: Helium
Flow rate: 1.5 mL/min
Injection size: 1 µL
Injection type: Split ratio, 200:1
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0 for the alpha tocopherol peak
Relative standard deviation: NMT 2.0% for the ratio of the alpha tocopherol peak area to the internal standard peak area
Analysis
Samples: Standard solution and Sample Solution
Calculate the percentage of d-alpha tocopherol (C29H50O2) in the portion of Vitamin E Polyethylene Glycol Succinate taken:
Result = (RU/RS) × (WS/WU) × 100
RU= internal standard ratio (peak area of alpha tocopherol/peak area of the internal standard) from the Sample solution
RS= internal standard ratio (peak area of alpha tocopherol/peak area of the internal standard) from the Standard solution
WS= weight of USP Alpha Tocopherol RS used to prepare the Standard solution (mg)
WU= weight of Vitamin E Polyethylene Glycol Succinate taken to prepare the Sample solution (mg)
Acceptance criteria: NLT 25.0%
SPECIFIC TESTS
• Optical Rotation, Specific Rotation 781S
[Note-This test identifies d-alpha tocopherol after saponification.]
Sample solution: Transfer 0.9 g of Vitamin E Polyethylene Glycol Succinate, molten at 60, to a suitable test tube fitted with a cap, and dissolve in 10.0 mL of alcohol. Place the tube in a heating block set at 100–105. [Note-Reflux the solution gently without emission of contents. ] When the sample is fully dissolved, add 2–3 pellets of sodium hydroxide, and continue to reflux for an additional 30 min. Remove the tube from the heat, and while contents are still hot, neutralize using phenolphthalein as the indicator by slowly adding 10 mL of a mixture of water and hydrochloric acid (1:1) until the pink color disappears. [Caution-Exothermic reaction. Allow the acid solution to trickle down the inside of the tube to prevent splashing.] Cool the tube, cap, and shake until contents are well mixed. Add 25.0 mL of heptane, cap, and shake for 1 min to ensure thorough mixing. Allow the tube to stand until two distinct layers are formed. Transfer the top layer to a clean, dry culture tube, then add 10.0 mL of water to the recovered solution. Cap, shake, and allow the layers to separate. Transfer the upper layer to a clean, dry tube. Add 10.0 mL of potassium ferricyanide solution, prepared by dissolving 2 g of potassium ferricyanide in 10.0 mL of 0.2 M sodium hydroxide, and replace the cap. Shake vigorously for 45 s, and allow the layers to separate for 30 min. If the top heptane layer is clear, proceed with the measurement for specific rotation; if not clear, dry over anhydrous sodium sulfate before proceeding with the test. [Note-Use the results of the test for Content of Alpha Tocopherol to calculate the specific rotation.]
Acceptance criteria: NLT +24.0
• Solubility in Water
Sample: 20 g of melted Vitamin E Polyethylene Glycol Succinate
Analysis: Place the Sample in a glass container on a magnetic stirrer. Immediately add 80 mL of boiling water while stirring. Allow to cool to room temperature with constant stirring.
Acceptance criteria: The solution becomes clear within 3 h.
• Acid Value
Sample: 1 g of Vitamin E Polyethylene Glycol Succinate
Analysis: Dissolve the Sample in 25 mL of a mixture of alcohol and ether (1:1) that has been neutralized to phenolphthalein with 0.1 N sodium hydroxide. Add 0.5 mL of phenolphthalein TS, and titrate with 0.10 N sodium hydroxide until the solution remains faintly pink after shaking for 30 s.
Acceptance criteria: NMT 0.027 mEq/g, equivalent to NMT 0.27 mL of 0.10 N sodium hydroxide
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers, and store protected from light.
• Labeling: The labeling indicates the d-alpha tocopherol content, expressed in mg/g.
• USP Reference Standards 11
USP Alpha Tocopherol RS
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
| WGK Germany | 2 |
| RTECS | TR1581200 |
Vitamin E Polyethylene Glycol Succinate (Vitamin E-TPGS; TPGS; Tocofersolan) (CAS: 9002-96-4) is a synthetic product. It is available as a off-white to pale yellow waxy Solid and is practically tasteless. Chemically, it is a mixture composed principally of monoesterified polyethylene glycol 1000, the diesterified polyethylene glycol 1000, free polyethylene glycol 1000, and free tocopherol.
Vitamin E Polyethylene Glycol Succinate has been widely used in pharmaceutical research, as a solubilizer, absorption promoter, emulsifier, plasticizer and carrier of water-insoluble or fat soluble drug delivery system, such as solid dispersion, carrier for ocular drug delivery, carrier for intranasal drug delivery, etc. As food additives.
Vitamin E Polyethylene Glycol Succinate is a water-soluble derivative of vitamin E, it is formed by reacting the carboxyl group of vitamin E succinate with the hydroxyl group of polyethylene glycol. Because it contains both vitamin E lipophilic group and polyethylene glycol hydrophilic long chain, it has good surfactant properties and water solubility, which can significantly increase the absorption of insoluble drugs in the gastrointestinal tract, increased bioavailability.
In recent years, many studies have found that TPGS not only can be used as pharmaceutical excipients, but also has many unique characteristics, as absorption enhancers and multidrug resistance reversal agents, TPGS can also be applied to prodrugs, micelles, liposomes, TPGS-copolymer carriers to improve the solubility, permeability and stability of the preparation, so as to achieve slow, controlled release and targeting effect, promote the absorption of drugs.
1. Pharmaceutical - Vitamin E-TPGS greatly enhances the absorption, bioavailability and effectiveness of pharmaceutical ingredients (APIs)
2. Dietery supplements - Vitamin E-TPGS is used to formulate supplements, especially those created to improve labsorption of vitamin E and other lipophilic nutrients and nutraceuticals
3. Food and Beverage - Vitamin E-TPGS can be employed to fortify foods and beverages, sports drinks, water and juices
4. Personal Care - Vitamin E-TPGS serves as an ethanol free, hypoallergenic, non-irritating emulsifier/excipient in personal care and cosmetic applications
5. Animal Nutrition Products - Vitamin E-TPGS supplies a highly absorbable and bioavailable vitamin E for animals that do not efficiently absorb traditional forms of vitamin E.
Vitamin E polyethylene glycol succinate is an esterified vitamin E (tocopherol) derivative primarily used as a solubilizer or emulsifying agent because of its surfactant properties. Structurally, it is amphipathic and hydrophilic, unlike the tocopherols, and therefore it is a water-soluble derivative that can be used in pharmaceutical formulations such as capsules, tablets, hot-melt extrusion, microemulsions, topical products, and parenterals. One of the most important applications is its use as a vehicle for lipid-based drug delivery formulations. It can also be used as a source of vitamin E. Vitamin E polyethylene glycol succinate has been characterized with respect to its mechanism of action and studied as a Pglycoprotein inhibitor.
Vitamin E polyethylene glycol succinate is incompatible with strong acids and strong alkalis.
GRAS listed. Included in the FDA Inactive Ingredients Database (ophthalmic solution or drops; oral capsules, solution, tablet; topical solution or drops). Included in the Canadian List of Acceptable Non-medicinal Ingredients.