Trimethyl Phosphonoacetate (TMPA) CAS 5927-18-4 Purity >99.0% (GC) Factory High Quality
Manufacturer Supply With High Quality, Commercial Production
Chemical Name: Trimethyl Phosphonoacetate CAS: 5927-18-4
| Chemical Name | Trimethyl Phosphonoacetate |
| Synonyms | TMPA; Dimethylphosphonoacetic Acid Methyl Ester; Methyl Dimethylphosphonoacetate; Phosphonoacetic Acid Trimethyl Ester; Methyl 2-(Dimethoxyphosphoryl)acetate |
| CAS Number | 5927-18-4 |
| CAT Number | RF-PI1247 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C5H11O5P |
| Molecular Weight | 182.11 |
| Boiling Point | 108℃/3 mmHg |
| Specific Gravity (20/20) | 1.27 |
| Refractive Index | n20/D 1.435~1.438 |
| Water Solubility | Slightly Miscible With Water |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Light Yellow Clear Liquid |
| Purity / Analysis Method | >99.0% (GC) |
| Moisture (K.F) | <0.50% |
| Single Impurity | <0.50% |
| Total Impurities | <1.00% |
| Clarity Test | Clear Without Suspended Substances |
| Acid Value (mgKOH/g) | <2.0 |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, 25kg/Barrel or 200kg/Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Trimethyl Phosphonoacetate (CAS: 5927-18-4) is mainly used in pharmaceutical industry as a pharmaceutical intermediate. Trimethyl phosphonoacetate is used in the preparation of sarin A by intramolecular Mannich-type reactions. Trimethyl Phosphonoacetate is involved in the Horner-Wadsworth-Emmons olefination with aldehydes and ketones to give acrylic esters. Trimethyl Phosphonoacetate is also involved in oxa-Michael reactions, prenylation of oxindoles and heterocyclization reactions.
-
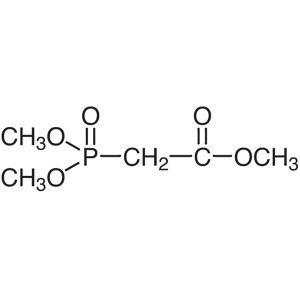
Trimethyl Phosphonoacetate (TMPA) CAS 5927-18-4...
-
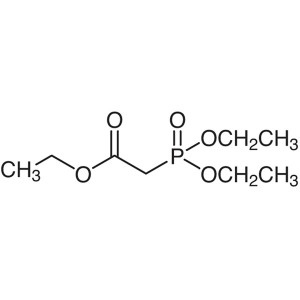
Triethyl Phosphonoacetate CAS 867-13-0 Purity >...
-
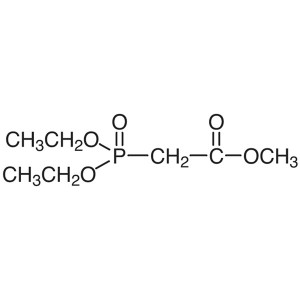
Methyl Diethylphosphonoacetate CAS 1067-74-9 Pu...
-
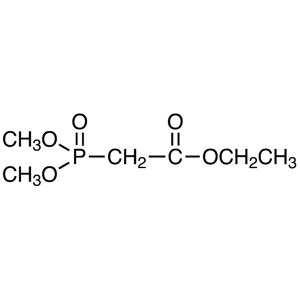
Ethyl Dimethylphosphonoacetate CAS 311-46-6 Pur...
-
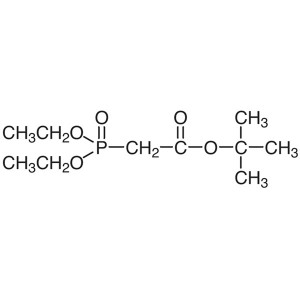
tert-Butyl Diethylphosphonoacetate CAS 27784-76...
-
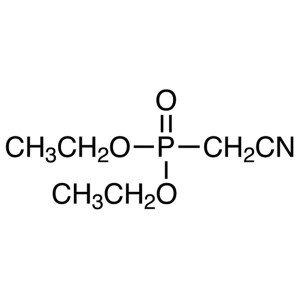
Diethyl Cyanomethylphosphonate CAS 2537-48-6 Pu...

