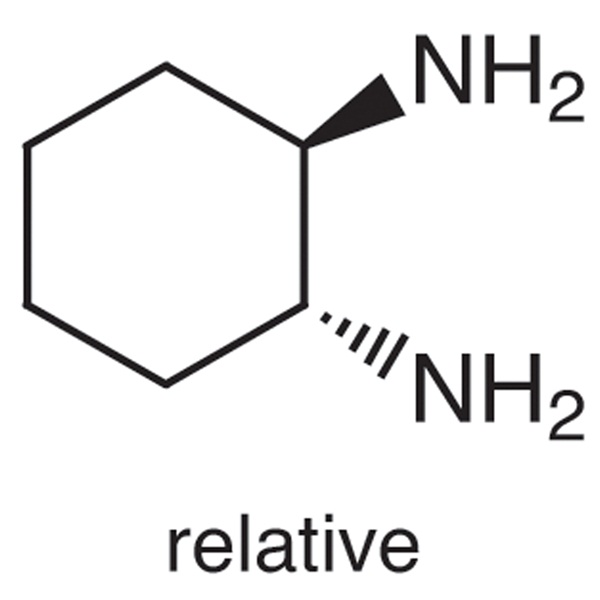
(±)-trans-1,2-Diaminocyclohexane CAS 1121-22-8 Purity ≥98.0% High Purity
Manufacturer Supply with High Purity and Stable Quality
Chemical Name: (±)-trans-1,2-Diaminocyclohexane
CAS: 1121-22-8
High Quality, Commercial Production
| Chemical Name | (±)-trans-1,2-Diaminocyclohexane |
| Synonyms | trans-1,2-Diaminocyclohexane; trans-1,2-Cyclohexanediamine |
| CAS Number | 1121-22-8 |
| CAT Number | RF-CC281 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C6H14N2 |
| Molecular Weight | 114.19 |
| Melting Point | 14.0-15.0℃ (lit.) |
| Boiling Point | 79.0-81.0℃/15 mmHg (lit.) |
| Density | 0.951 g/mL at 25℃ (lit.) |
| Refractive Index | n20/D 1.489 (lit.) |
| Solubility | Soluble in Water |
| Sensitivity | Air Sensitive, Hygroscopic |
| Shipping Condition | Shipped Under Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless or Yellowish Liquid |
| Purity | ≥98.0% (GC) |
| Water | ≤1.00% |
| Total Impurities | ≤2.00% |
| Test Standard | Enterprise Standard |
| Usage | Chiral Compounds; Pharmaceutical Intermediates |
Package: Bottle, 25kg/Barrel, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of (±)-trans-1,2-Diaminocyclohexane (CAS: 1121-22-8) with high quality.
(±)-trans-1,2-Diaminocyclohexane (CAS: 1121-22-8) is an organic compound with the formula C6H10(NH2)2. This diamine is a building block for C2-symmetric ligands that are useful in asymmetric catalysis. (±)-trans-1,2-Diaminocyclohexane is employed in the synthesis of macrocyclic [3+3] hexa Schiff base. It also plays an important role in the preparation of multidentate ligands, chiral auxiliaries and chiral stationary phases. Further, it is used to prepare [2+2] macrocyclization by reacting with aliphatic dialdehydes. In addition to this, it acts as a chiral ligand, which finds application in asymmetric catalysis.
-
1,2-Diaminocyclohexane (mixture of cis and tran...
-
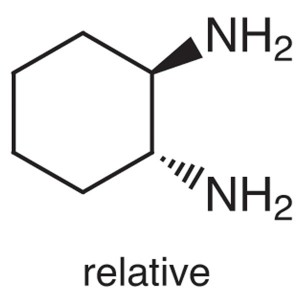
(±)-trans-1,2-Diaminocyclohexane CAS 1121-22-8 ...
-
cis-1,2-Diaminocyclohexane CAS 1436-59-5 Purity...
-
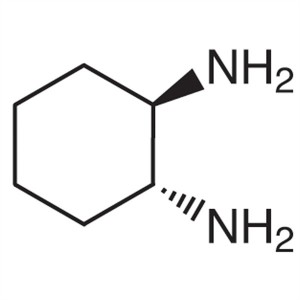
(1R,2R)-(-)-1,2-Diaminocyclohexane CAS 20439-47...
-
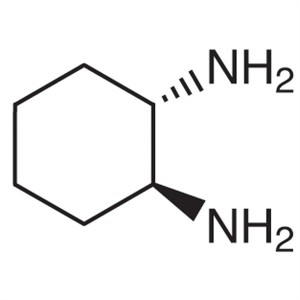
(1S,2S)-(+)-1,2-Diaminocyclohexane CAS 21436-03...
-
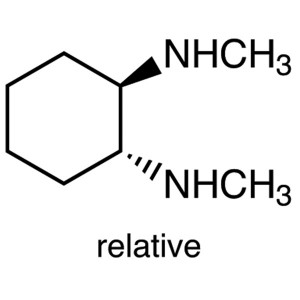
trans-N,N’-Dimethylcyclohexane-1,2-diamin...
-
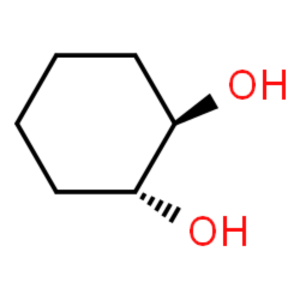
(1R,2R)-trans-1,2-Cyclohexanediol CAS 1072-86-2...
-
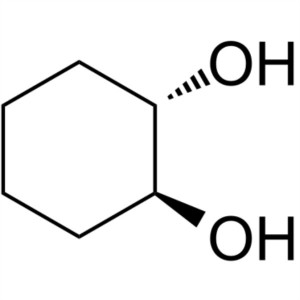
(1S,2S)-trans-1,2-Cyclohexanediol CAS 57794-08-...
-
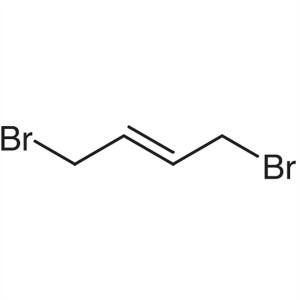
trans-1,4-Dibromo-2-butene CAS 821-06-7 Purity ...
-
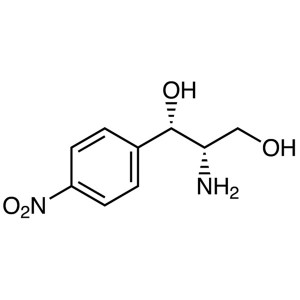
(1S,2S)-(+)-2-Amino-1-(4-nitrophenyl)-1,3-propa...
-
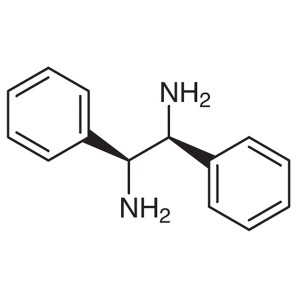
(1S,2S)-(-)-1,2-Diphenylethylenediamine CAS 298...
-
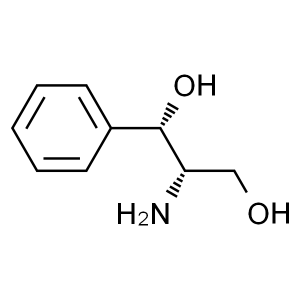
(1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol CA...
-
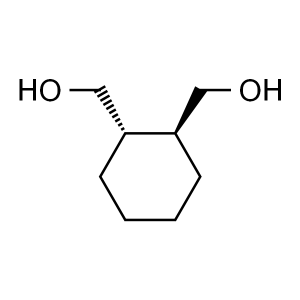
(1S,2S)-1,2-Cyclohexanedimethanol CAS 3205-34-3...
-
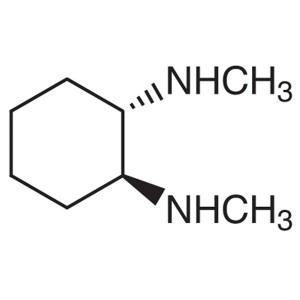
(1S,2S)-N,N’-Dimethyl-1,2-Cyclohexanediam...
-
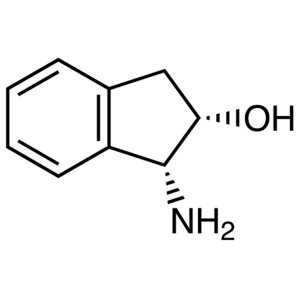
(1R,2S)-(+)-1-Amino-2-indanol CAS 136030-00-7 P...
-
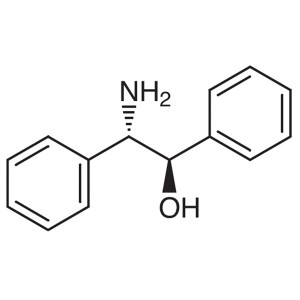
(1R,2S)-(-)-2-Amino-1,2-Diphenylethanol CAS 231...