Tranexamic Acid CAS 1197-18-8 Assay 99.0~101.0%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Tranexamic Acid (CAS: 1197-18-8) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Tranexamic Acid, Please contact: alvin@ruifuchem.com
| Chemical Name | Tranexamic Acid |
| Synonyms | trans-4-(Aminomethyl)cyclohexanecarboxylic Acid; trans-Tranexamic Acid; TAMCHA; AMCHA; HAKU; AMCA; t-AMCHA; trans-Amcha |
| Stock Status | In Stock, Production Capacity 100 Metric Tons per Year |
| CAS Number | 1197-18-8 |
| Molecular Formula | C8H15NO2 |
| Molecular Weight | 157.21 g/mol |
| Melting Point | >300℃(lit.) |
| Stability | Hygroscopic |
| Water Solubility | Soluble in Water, Almost Transparency |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories | Pharmaceutical Intermediates |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White Crystalline Powder | Complies |
| Solubility | Soluble in Water and Glacial Acetic Acid, Almost Not Soluble in Ethanol and Acetone (96%) |
Complies |
| IR Spectrum | In Concordance with Tranexamic Acid CRS | Complies |
| pH | 7.0~8.0 | 7.32 |
| Loss on Drying | <0.50% | 0.06% |
| Residue on Ignition | <0.10% | 0.02% |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Chloride (Cl) | ≤140ppm | <140ppm |
| Arsenic (As) | ≤2ppm | <2ppm |
| Assay | 99.0~101.0% | 99.84% |
| Related Substances | ||
| Impurity A | <0.10% | 0% |
| Impurity B | <0.20% | 0.0815% |
| Impurity C | <0.10% | 0.0089% |
| Impurity D | <0.10% | 0.0063% |
| Total Impurities | <0.20% (Except A,B) | 0.015% |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Fluorinated Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
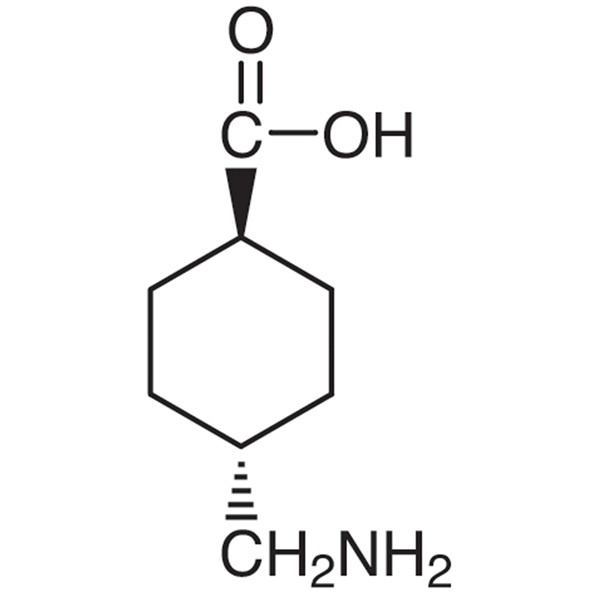
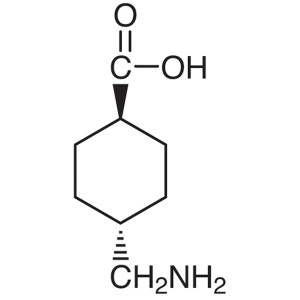
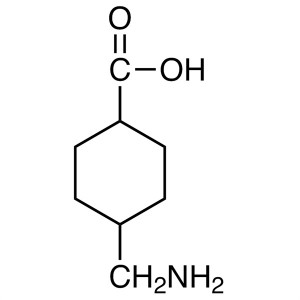
Tranexamic Acid
C8H15NO2 157.2
trans-4-(Aminomethyl)cyclohexanecarboxylic acid;
Cyclohexanecarboxylic acid, 4-(aminomethyl)-, trans [1197-18-8].
DEFINITION
Tranexamic Acid contains NLT 99.0% and NMT 101.0% of C8H15NO2, calculated on the dried basis.
IDENTIFICATION
• Infrared Absorption <197K>
ASSAY
• Procedure
Sample solution: 140 mg of Tranexamic Acid in 20 mL of glacial acetic acid
Titrimetric system
(See Titrimetry <541>.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Potentiometric
Analysis
Sample: Sample solution
Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Carry out a blank titration.
Each mL of 0.1 N perchloric acid is equivalent to 15.72 mg of C8H15NO2.
Acceptance criteria: 99.0%-101.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition <281>: NMT 0.1%; 1-g sample is used
• Heavy Metals, Method II <231>: NMT 10 ppm
• Chloride and Sulfate, Chloride <221>: A 0.51-g portion shows no more chloride than corresponds to 0.1 mL of 0.020 N hydrochloric acid (0.014%).
Organic Impurities
• Procedure
Mobile phase: Dissolve 11.0 g of anhydrous monobasic sodium phosphate in 500 mL of water, and add 5 mL of triethylamine, followed by 1.4 g of sodium lauryl sulfate. Adjust with diluted phosphoric acid (10% w/w) to a pH of 2.5, and dilute with water to 600 mL. Mix this solution with 400 mL of methanol.
System suitability solution: 0.2 mg/mL of USP Tranexamic Acid RS and 0.002 mg/mL of USP Tranexamic Acid Related Compound C RS in water
Standard solution: 50 µg/mL of USP Tranexamic Acid RS in water
Sample solution: 10 mg/mL of Tranexamic Acid in water
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection size: 20 µL
Run time: 3 times the retention time of tranexamic acid
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 2.0 between Tranexamic Acid and 0.002 mg/mL of tranexamic acid related compound C
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Tranexamic Acid taken:
Result = (rU/rS) × (CS/CU) × (0.1F)
rU = peak response for each impurity from the Sample solution
rS = peak response for tranexamic acid from the Standard solution
CS = concentration of USP Tranexamic Acid RS in the Standard solution (µg/mL)
CU = concentration of Tranexamic Acid in the Sample solution (mg/mL)
F = relative response factor (see Impurity Table 1)
Acceptance criteria
Individual impurities: See Impurity Table 1.
Total impurities: NMT 0.2%
[Note-Disregard any peak less than 0.025%.]
Impurity Table 1
Name Relative Retention Time Relative Response Factor Acceptance Criteria, NMT (%)
Tranexamic acid related compound Aa 2.1 1 0.1
Tranexamic acid related compound Bb 1.5 1.2 0.2
Tranexamic acid related compound Cc 1.1 0.005 0.1
Tranexamic Acid 1.0 1.0 —
Tranexamic acid related compound Dd 1.3 0.006 0.1
a trans,trans-4,4¢-(Iminodimethylene)di(cyclohexanecarboxylic)acid.
b cis-4-(Aminomethyl)cyclohexanecarboxylic acid.
c (RS)-4-(Aminomethyl) cyclohex-1-enecarbocylic acid.
d 4-Aminomethyl benzoic acid.
SPECIFIC TESTS
• Loss on Drying <731>: Dry 1.00 g at 105 under vacuum for 2 h. It loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage: Preserve in tight containers, and store at a temperature not exceeding 30.
• USP Reference Standards <11>
USP Tranexamic Acid RS Click to View Structure
USP Tranexamic Acid Related Compound C RS
(RS)-4-(Aminomethyl)cyclohex-1-enecarbocylic acid.
C8H13NO2 155
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Hazard Symbols Xi - Irritant
Risk Codes 36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
S37/39 - Wear suitable gloves and eye/face protection
WGK Germany 2
RTECS GU8400000
HS Code 2922491100
Hazard Class IRRITANT
Toxicity LD50 in mice, rats (mg/kg): 1500, 1200 i.v. (Melander)
Tranexamic Acid (CAS: 1197-18-8) is a synthetic derivative of the amino acid lysine.Tranexamic acid (commonly marketed in tablet form as Lysteda and in IV form as Cyklokapron in the U.S. and as Transamin,Transcam in Asia, and Espercil in South America) is often prescribed for excessive bleeding. Tranexamic acid is an antifibrinolytic that competitively inhibits the activation of plasminogen to plasmin, a molecule responsible for the degradation of fibrin. Fibrin is the basic framework for the formation of a blood clot in hemostasis.Tranexamic acid has roughly 8 times the antifibrinolytic activity of an older analogue, ε-aminocaproic acid. It was first patented in 1957 and received its initial US approval in 1986. Tranexamic Acid Applicaiton Used as whitening cosmetics It is widely used clinically in the fields of surgery, internal medicine, urology, obstetrics and gynecology, etc. to treat various bleeding diseases and abnormal bleeding during surgery, etc. It is considered as a hemostatic medicine, which is mainly used for hemostasis after major surgery and postpartum. Of course, hemostasis is probably the minimum reason why many toothpastes are added to hope to have an effect.
Tranexamic Acid (CAS: 1197-18-8)
Heart surgery
Commonly used in cardiac surgery, both with and without cardiopulmonary bypass. It replaces aprotinin.
Menstrual bleeding
Used as firstline nonhormonal treatment of dysfunctional uterine bleeding, and heavy bleeding associated with uterine fibroids. A recent study showed patients treated with tranexamic acid are more likely to develop thrombosis and necrosis in their fibroids, and may result in pain and fever.
Whitening effect
Clinical studies for years have proved that tranexamic acid can dilute spot effectively and rapidly, which helps to demonstrate a perfect white and bright skin. The spot-removing effect of tranexamic acid is about 50 times over Vitamin C, and 10 times over AHA. The concentration limit of usage is 2%-3%, and in cosmetics the amount is around 0.5%
1. Good stability
Compared with traditional whitening ingredients, tranexamic acid has high stability, acid and alkali resistance, and is not easily affected by temperature environment; No carrier protection, no transmission system damage, no stimulation.
2. The skin system is easy to absorb
It is especially suitable for lightening spots, whitening and balancing the overall skin tone. In addition to lightening the spots, tranexamic acid can also improve the transparency of overall skin color and the dullness of local skin mass.
3. It can fade black spots, yellow freckles, acne marks, etc
Black spots are caused by UV damage and skin aging and other factors. Clotting acid can inhibit the activity of tyrosinase and melanocytes, reduce the production of melanin from the basal layer of epidermis, and also has the effect of removing red spots.
4. High safety
The highest concentration in cosmetics is 2%~3%.
5. Whitening effect
Tranexamic Acid is suitable to all kinds of skin for removing pigmentation, whitening skin and reducing spots, such as: Pigmentation after sun exposure; Dark spots; Sensitive skin; Acne and inflammation; Postoperative care after laser, pulsed light treatment.