Titanium(IV) Chloride (TiCl4) CAS 7550-45-0 Purity >99.9%
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Titanium(IV) Chloride (Titanium Tetrachloride) (CAS: 7550-45-0) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | Titanium(IV) Chloride |
| Synonyms | Titanium Tetrachloride; Titanic Chloride; TiCl4 |
| CAS Number | 7550-45-0 |
| CAT Number | RF-PI2232 |
| Stock Status | In Stock, Production Capacity 50000MT/Year |
| Molecular Formula | TiCl4 |
| Molecular Weight | 189.67 |
| Melting Point | -25℃(lit.) |
| Boiling Point | 135.0~136.0℃(lit.) |
| Specific Gravity (20/20℃) | 1.728 g/cm3 |
| Sensitive | Moisture Sensitive, Heat Sensitive |
| Storage Temperature | Room Temperature, Flammable Area |
| Water Solubility | Reacts |
| Hydrolytic Sensitivity | 8: Reacts Rapidly With Moisture, Water, Protic Solvents |
| Stability | Stable. Reacts With Water. Incompatible With Moisture, Ammonia, Amines, Alcohols, Potassium and Other Chemically Active Metals |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless to Pale Yellow Clear Liquid, No Impurity |
| Purity / Analysis Method | >99.9% |
| Color | <5mg/L (K2Cr2O7) |
| Impurity Analysis (wt%) | |
| Tetrachlorosilane (SiCl4) | ≤0.01% |
| Ferric Chloride (FeCl3) | ≤0.002% |
| Vanadium(v)trichloride Oxide (VoCl3) | ≤0.0024% |
| ICP Major Analysis | Confirms Titanium Component Confirmed |
| X-Ray Diffraction | Conforms to Structure |
| Test Standard | Enterprise Standard |
Package: Fluorinated Bottle, 25kg/Drum, 250kg/Drum or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Titanium(IV) Chloride (Titanium Tetrachloride) (CAS: 7550-45-0) is widely used in production of metal titanium, titanium dichloride, organic titanium compounds, titanate and smog bomb; important composition of producing acryl and ethylene catalyst. Titanium(IV) Chloride, at room temperature, it can easily occur smoke when exposed to the air. It has characteristic odor and acid taste. Heated Titanium(IV) Chloride could break into chlorides HCl and some solid substance. It would be used for the smoke screen. It is also widely used in military field. Titanium(IV) Chloride is used as an intermediate in the manufacture of titanium metal, titanium dioxide, titanous chloride pigments, iridescent glass, and artificial pearls and as a starting material for a variety of organic and inorganic titanium compounds. Titanium(IV) Chloride is also used as a dye, a polymerization catalyst, and as a catalyst in many organic syntheses because of it acidity and oxophilicity in many applications in the chemical industry. The conversion of tetrachloride to titanium metal takes place by the reduction of chloride with magnesium which yields titanium metal and magnesium chloride.
Hazard: Toxic by inhalation, strong irritant to skin and tissue. Health Hazard Titanium tetrachloride is a highly corrosive, acute irritant to the skin, eyes, mucous membranes and the respiratory tract. It is capable of causing death or permanent injury due to exposures encountered in normal use. Even short contact may lead to eye inflammation which may result in corneal opacities.
Fire Hazard: Material will react with water to produce hydrochloric acid. Titanium tetrachloride may ignite other combustible materials (e.g., wood, oil, etc.). Flammable, poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Reacts strongly with water to release hydrochloric acid and heat. Avoid water, moist air. Stable in concentrated aqueous solutions. Avoid contact with moisture; the chemical absorbs moisture from air and evolves dense white fumes.
-

Titanium(IV) Chloride (TiCl4) CAS 7550-45-0 Pur...
-
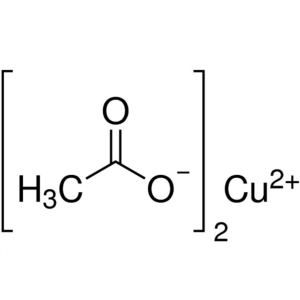
Cupric Acetate Anhydrous CAS 142-71-2 Purity >9...
-

Copper(I) Bromide Cuprous Bromide CAS 7787-70-4...
-

Copper(I) Iodide CAS 7681-65-4 Purity >99.0% (C...
-

Sodium Thiosulfate CAS 7772-98-7 Purity >99.0% ...
-
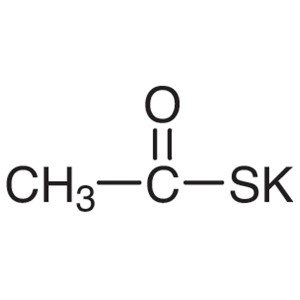
Potassium Thioacetate CAS 10387-40-3 Purity >98...
-

Ferrous Sulfate Heptahydrate CAS 7782-63-0 Assa...
-

Ferrous Sulfate Monohydrate CAS 13463-43-9 Puri...
-
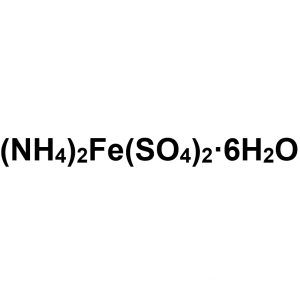
Ammonium Iron(II) Sulfate Hexahydrate CAS 7783-...
-
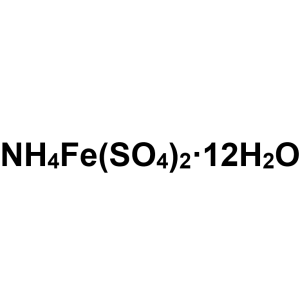
Ammonium Iron(III) Sulfate Dodecahydrate CAS 77...
-

Cerium(III) Chloride Heptahydrate CAS 18618-55-...
-

Copper(I) Chloride CAS 7758-89-6 Cuprous Chlori...
-
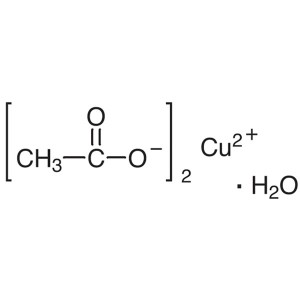
Copper(II) Acetate Monohydrate CAS 6046-93-1 Pu...

