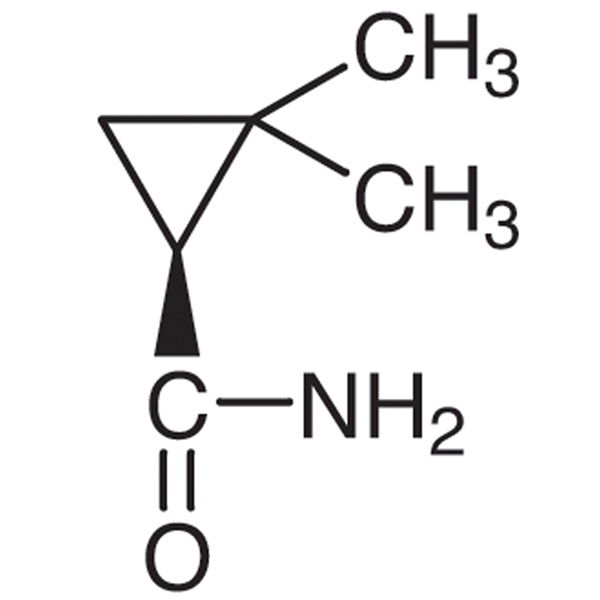
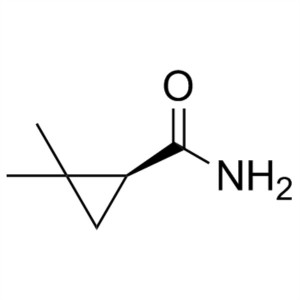
(S)-(+)-2,2-Dimethylcyclopropanecarboxamide CAS 75885-58-4 Purity >98.0% (GC) Cilastatin Sodium Intermediate
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of (S)-(+)-2,2-Dimethylcyclopropanecarboxamide (CAS: 75885-58-4) with high quality, intermediate of Cilastatin Sodium (CAS: 81129-83-1). Formulations containing Cilastatin in combination with Imipenem have been used to treat susceptible bacterial infections.
Ruifu Chemical has been supplying pharmaceutical intermediates and APIs, fine chemicals more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Cilastatin Sodium intermediates, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | (S)-(+)-2,2-Dimethylcyclopropanecarboxamide |
| Synonyms | SDMCA; (S)-(+)-DMCPA; S-(+)-2,2-Dimethylcyclopropane Carboxamide; (S)-2,2-Dimethylcyclopropane-1-Carboxamide |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 75885-58-4 |
| Molecular Formula | C6H11NO |
| Molecular Weight | 113.16 g/mol |
| Melting Point | 135.0 to 140.0℃ |
| Density | 1g/cm3 |
| Solubility | Soluble in Methanol |
| Storage Temperature | Store Long-Term in a Cool and Dry Place |
| COA & MSDS | Available |
| Origin of Product | Shanghai, China |
| Product Categories |
Pharmaceutical Intermediates; Chiral Building Blocks |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White or Off-White Crystalline Powder | Conforms |
| Melting Point | 135.0~140.0℃ | 135.0~137.0℃ |
| Specific Rotation [a]20/D | +78.0° to +88.0° (C=1 in Methanol) | +82.3° |
| Water by Karl Fischer | <0.50% | 0.25% |
| Sulphate Ash | <0.20% | 0.08% |
| Purity / Analysis Method | >99.0% (GC) | 99.9% |
| Chiral Purity (e.e.) | >99.0% (GC) | 99.8% |
| 1H NMR Spectrum | Conforms to Structure | Conforms |
| Conclusion | The product has been tested and complies with the given specifications | |
| Application | Intermediate of Cilastatin Sodium (CAS: 81129-83-1) | |
Package: Fluorinated bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Risk Codes
R22 - Harmful if swallowed
R36/37/38 - Irritating to eyes, respiratory system and skin.
Safety Description
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36 - Wear suitable protective clothing.
WGK Germany 3
HS Code 2924 2990.99
Hazard Note Irritant
(S)-(+)-2,2-Dimethylcyclopropanecarboxamide (CAS: 75885-58-4), intermediate of Cilastatin (CAS: 82009-34-5) and Cilastatin Sodium (CAS: 81129-83-1).
Cilastatin has antibacterial effects on Gram-positive and negative aerobic and anaerobes. The antibacterial spectrum includes Streptococcus, Staphylococcus aureus, Escherichia coli, Klebsiella, some strains of Acinetobacter, Haemophilus bacillus, Proteus, Serratia, Pseudomonas aeruginosa, etc. Clinically, Cilastatin is mainly used for respiratory tract infections, biliary tract infections, urinary system and abdominal cavity infections, skin and soft tissues, bones and joints, gynecological infections caused by gram-positive bacteria, negative bacteria, and anaerobic bacteria.
Cilastatin was designed to inhibit renal metabolism of Imipenem and prolong its half-life. Formulations containing Cilastatin in combination with Imipenem have been used to treat susceptible bacterial infections.
When administered alone, Imipenem is metabolised in the kidneys by dehydropeptidase-I, an enzyme in the brush border of the renal tubules, to inactive, nephrotoxic metabolites, with only about 5 to 40 or 45% of a dose excreted in the urine as unchanged active drug. Cilastin inhibits the metabolism of imipenem. When given with cilastatin about 70% of an intravenous dose of Imipenem is recovered unchanged in the urine within 10 hours. Cilastatin is also excreted mainly in the urine, the majority as unchanged drug and about 12% as N-Acetyl Cilastatin. Less than 1% of Imipenem is excreted via the bile in the faeces.
-
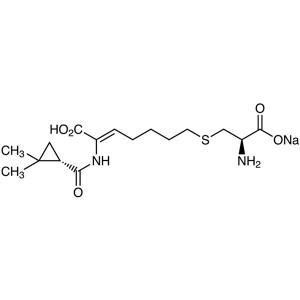
Cilastatin Sodium CAS 81129-83-1 Assay 98.0~101.5%
-
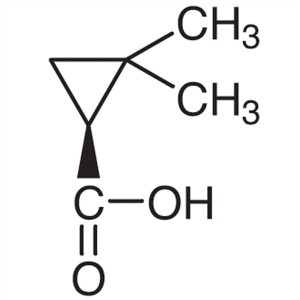
(S)-(+)-2,2-Dimethylcyclopropanecarboxylic Acid...
-
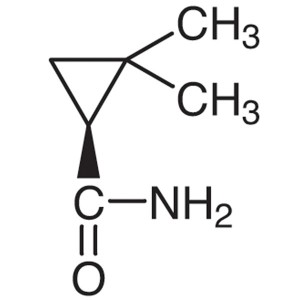
(S)-(+)-2,2-Dimethylcyclopropanecarboxamide CAS...
-
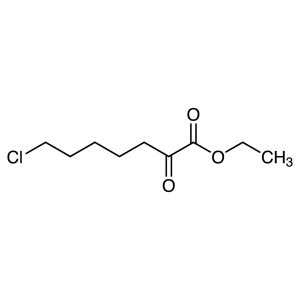
Ethyl 7-Chloro-2-Oxoheptanoate CAS 78834-75-0 P...
-
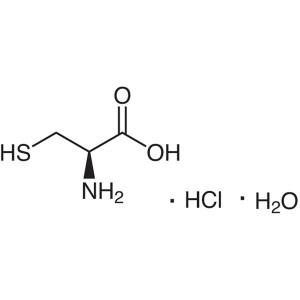
L-Cysteine Hydrochloride Monohydrate CAS 7048-0...
-
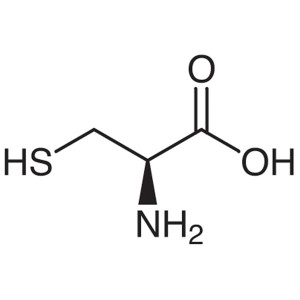
L-Cysteine CAS 52-90-4 (H-Cys-OH) Assay 98.5~10...