(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol CAS 127852-28-2 Purity >99.0% (GC) Aprepitant Intermediate
Leading manufacturer and supplier
Intermediates of Aprepitant (CAS: 170729-80-3)
4-Benzyl-2-Hydroxymorpholin-3-one CAS 287930-73-8
(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol CAS 127852-28-2
Please contact: alvin@ruifuchem.com
| Chemical Name | (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol |
| Synonyms | (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethyl Alcohol; (R)-3,5-Bis(trifluoromethyl)-α-Methylbenzyl Alcohol |
| CAS Number | 127852-28-2 |
| CAT Number | RF-CC302 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C10H8F6O |
| Molecular Weight | 258.16 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White to Off-White Powder |
| Purity / Analysis Method | >99.0% (GC) |
| Melting Point | 53.0~57.0℃ |
| Optical Rotation | +26.0° ~ +29.0° (C=1 IN Acetonitrile) |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Usage | Intermediate of Aprepitant (CAS: 170729-80-3) |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol (CAS: 127852-28-2) is used as pharmaceutical intermediate, chiral synthesis for organic reactions, Aprepitant (CAS: 170729-80-3) intermediate. Aprepitant is an antiemetic chemical compound. Aprepitant is used for the prevention of acute and delayed chemotherapy-induced nausea and vomiting(CINV) and for prevention of postoperative nausea and vomiting. It was first successfully developed by Merck Company (German). In March 2003, the US Food and Drug Administration approved it for being used in the treatment of chemotherapy vomiting.
-
![(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol CAS 127852-28-2 Purity >99.0% (GC) Aprepitant Intermediate](https://www.ruifuchem.com/uploads/R-1-35-Bistrifluoromethylphenylethanol-CAS-127852-28-2-300x300.jpg)
(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol C...
-
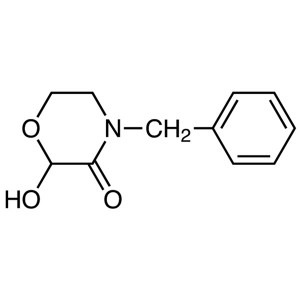
4-Benzyl-2-Hydroxymorpholin-3-one CAS 287930-73...
-
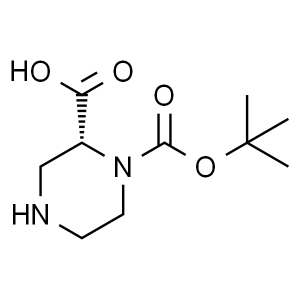
(R)-1-Boc-Piperazine-2-Carboxylic Acid CAS 2787...
-
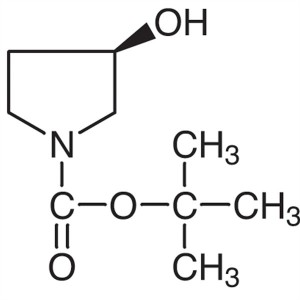
(R)-1-Boc-3-Hydroxypyrrolidine CAS 109431-87-0 ...
-
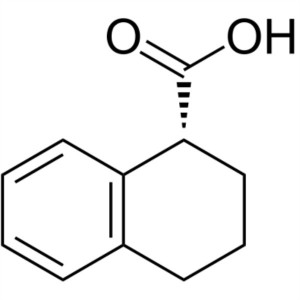
(R)-1,2,3,4-Tetrahedro-1-Naphthoic Acid CAS 233...
-
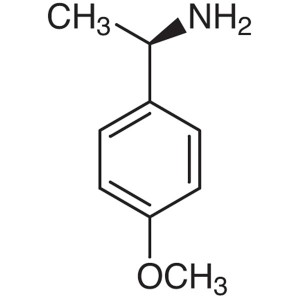
(R)-(+)-1-(4-Methoxyphenyl)ethylamine CAS 22038...
-
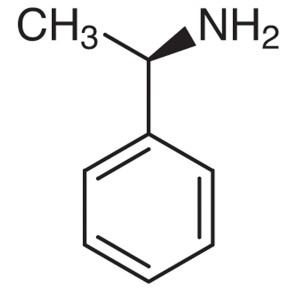
(R)-(+)-1-Phenylethylamine ; (R)-(+)-α-Methylbe...
-
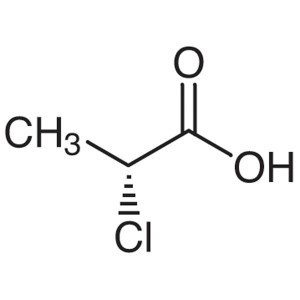
(R)-(+)-2-Chloropropionic Acid CAS 7474-05-7 Pu...
-
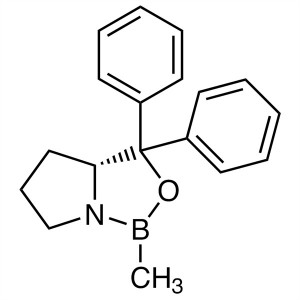
(R)-(+)-2-Methyl-CBS-oxazaborolidine; (R)-Me-CB...
-
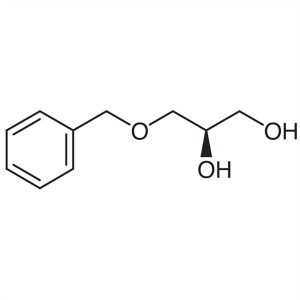
(R)-(+)-3-Benzyloxy-1,2-Propanediol CAS 56552-8...
-
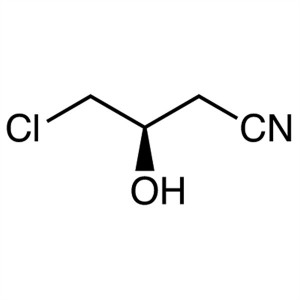
(R)-(+)-4-Chloro-3-Hydroxybutyronitrile CAS 843...
-
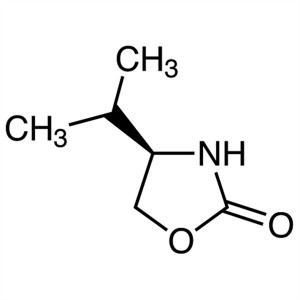
(R)-(+)-4-Isopropyl-2-Oxazolidinone CAS 95530-5...
-
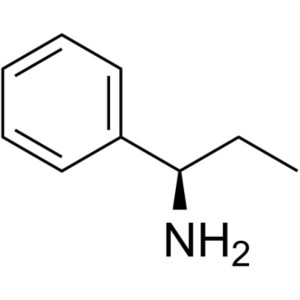
(R)-(+)-1-Phenylpropylamine CAS 3082-64-2 Purit...
-
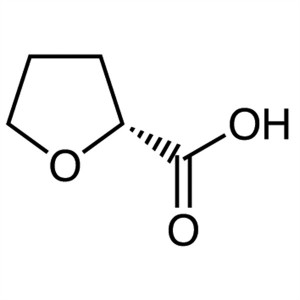
(R)-(+)-2-Tetrahydrofuroic Acid CAS 87392-05-0 ...
-
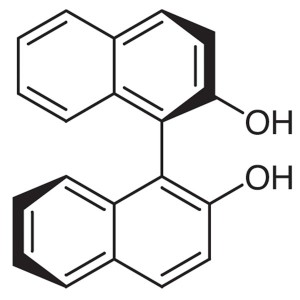
(R)-(+)-1,1′-Bi-2-naphthol CAS 18531-94-7...
-
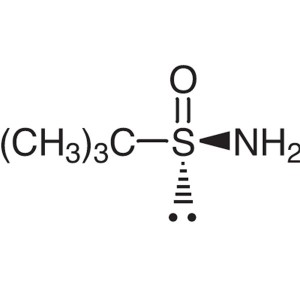
(R)-(+)-tert-Butylsulfinamide CAS 196929-78-9 P...

![(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol CAS 127852-28-2 Purity >99.0% (GC) Aprepitant Intermediate Featured Image](https://www.ruifuchem.com/uploads/R-1-35-Bistrifluoromethylphenylethanol-CAS-127852-28-2.jpg)