PCC Pyridinium Chlorochromate CAS 26299-14-9 Assay ≥98.5% Factory
Manufacturer Supply, High Purity, Commercial Production
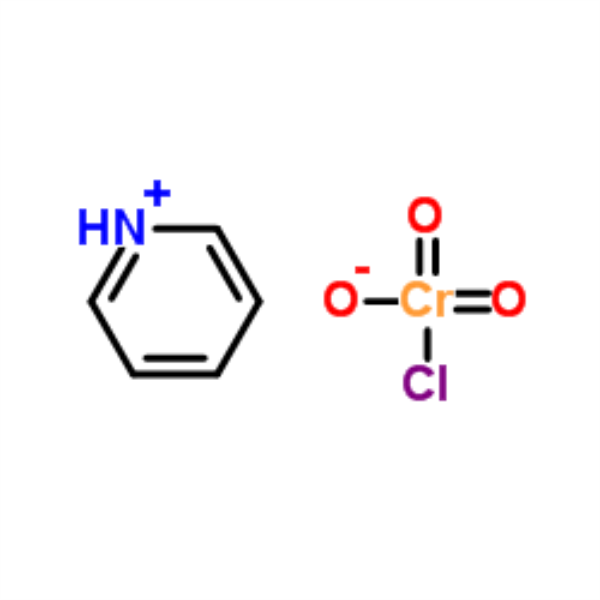
Chemical Name: Pyridinium Chlorochromate (PCC)
CAS: 26299-14-9
| Chemical Name | Pyridinium Chlorochromate |
| Synonyms | PCC |
| CAS Number | 26299-14-9 |
| CAT Number | RF-PI535 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C5H6N·ClCrO3 |
| Molecular Weight | 215.55 |
| Melting Point | 205.0 to 208.0℃ (lit.) |
| Solubility | Soluble in Acetone, Benzene, Dichloromethane, Acetonitrile |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Orange Crystalline Powder |
| Assay | ≥98.5% |
| Loss on Drying | ≤1.0% |
| Heavy Metals (as Pb) | ≤20ppm |
| Test Standard | Enterprise Standard |
| Usage | Oxidizing Agent |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture.


Pyridinium Dichromate (PDC) or Pyridinium Chlorochromate (PCC) in anhydrous media such as dichloromethane oxidizes primary alcohols to aldehydes while avoiding overoxidation to carboxylic acids. PCC was first a new selective oxidant discovered by E.J. Corey in 1975 after developing research. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective. Pyridinium Chlorochromate is used as an oxidizing agent to convert primary and secondary alcohols to aldehydes and ketones respectively. It is involved in the preparation of cyclohexanone, (-)-pulegone and lactones. It plays an important role in the selective mono-oxidation of xylenes to tolualdehydes and arylhydroxyamines to nitroso compounds. Furthermore, it is utilized as an oxidant for amino acids, L-cystine, aniline, cycloalkanols, vicinal and non-vicinal diols as well as in the Babler oxidation reaction.