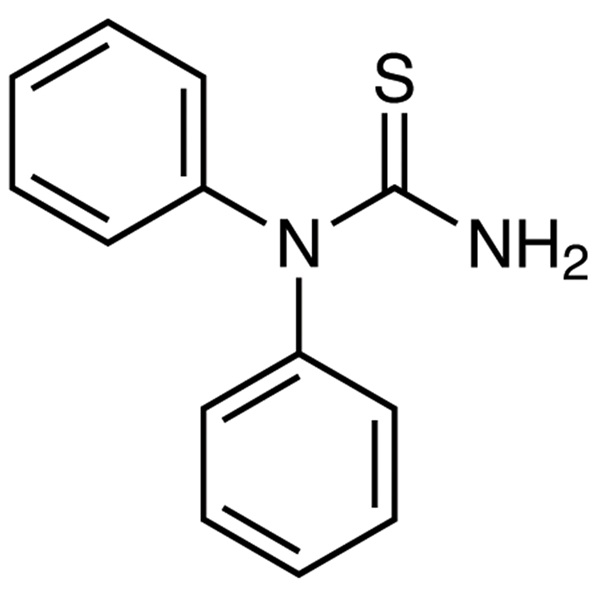
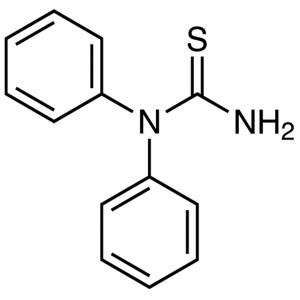
N,N-Diphenylthiourea CAS 3898-08-6 Purity >98.0% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading supplier of N,N-Diphenylthiourea (CAS: 3898-08-6) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. If you are interested in this product, please send detailed information includes CAS number, product name, quantity to us. Please contact: alvin@ruifuchem.com
| Chemical Name | N,N-Diphenylthiourea |
| Synonyms | 1,1-Diphenyl-2-Thiourea |
| CAS Number | 3898-08-6 |
| CAT Number | RF-PI2274 |
| Stock Status | In Stock, Production Capacity 15MT/Month |
| Molecular Formula | C13H12N2S |
| Molecular Weight | 228.31 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Light Yellow Powder |
| Purity / Analysis Method | >98.0% (HPLC) |
| Purity / Analysis Method | >98.0% (Nonaqueous Titration) |
| Melting Point | 210.0~215.0℃ |
| Loss on Drying | <0.50% |
| Total Impurities | <2.00% |
| Test Standard | Enterprise Standard |
| Usage | Pharmaceutical Intermediates |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


N,N-Diphenylthiourea, also known as 1,1-Diphenyl-2-Thiourea, (DT) (CAS: 3898-08-6) is a compound that contains a thiourea moiety. It is an organic compound that can be synthesized by the reaction of benzene and sodium thiosulfate. The DT molecule has a disulfide bond between its two sulfur atoms. DT crystallizes in the monoclinic system with space group C2/m and lattice constants a = 5.8331 A, b = 10.6263 A, c = 8.7691 A and β = 99.15°. DT has been used as a reagent for analytical chemistry to identify certain compounds, such as chloride ions, by adding DT to the mixture and observing how it reacts with the chloride ions to form DT chloride (DTCl). DT also forms oxidation products when exposed to air or light, which can be used to identify it using chromatography or spectroscopy techniques.
-
Chloromethyl Ethyl Ether CAS 3188-13-4 Assay ≥9...
-
Hemimellitic Acid CAS 569-51-7 Purity >98.0% (H...
-
4-Bromo-3,5-Dimethylphenol CAS 7463-51-6 Purity...
-
2-Methylthiazole CAS 3581-87-1 Purity >99.0% (G...
-
2,2,6,6-Tetramethylpiperidine CAS 768-66-1 Puri...
-
4-Iodobenzyl Alcohol CAS 18282-51-4 Purity >99....